UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of incorporation) |
(Commission File Number) | (IRS Employer Identification No.) |
|
|
||
| (Address of principal executive offices) | (Zip Code) |
(
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) | |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) | |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) | |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| The Capital Market | ||||
| The Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR §240.12b-2).
Emerging growth company
If an emerging growth company, indicate by check
mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards provided pursuant to Section 13(a) of the Exchange Act.
Item 7.01 Regulation FD
On November 20, 2025, Pasithea Therapeutics Corp. (the “Company”) issued the November 20 Press Release (as defined below). A copy of the November 20 Press Release is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is hereby incorporated by reference herein.
On November 21, 2025, the Company issued the November 21 Press Release (as defined below). A copy of the November 21 Press Release is furnished as Exhibit 99.2 to this Current Report on Form 8-K and is hereby incorporated by reference herein.
On November 24, 2025, the Company issued the November 24 Press Release (as defined below). A copy of the November 24 Press Release is furnished as Exhibit 99.3 to this Current Report on Form 8-K and is hereby incorporated by reference herein.
On November 25, 2025, the Company issued the November 25 Press Release (as defined below). A copy of the November 25 Press Release is furnished as Exhibit 99.4 to this Current Report on Form 8-K and is hereby incorporated by reference herein.
The information in this Current Report on Form 8-K under Item 7.01, including the information contained in Exhibit 99.1, Exhibit 99.2, Exhibit 99.3, and Exhibit 99.4, is being furnished to the Securities and Exchange Commission (the “SEC”), and shall not be deemed to be “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, and shall not be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by a specific reference in such filing.
Item 8.01 Other Events.
On November 20, 2025, the Company issued a press release (the “November 20 Press Release”) relating to positive interim Phase 1 data from its ongoing first-in-human clinical trial of PAS-004 in patients with advanced solid tumors driven by MAPK pathway alterations, including documented RAS, NF1 or RAF mutations, and in patients who have failed prior BRAF/MEK inhibition.
The Company released the below interim Phase 1 results for PAS-004 in the Press Release:
Initial Signals of Clinical Activity
Among 21 efficacy evaluable patients (as per RECIST1.1):
| ● | Partial Response: | |
| ○ | A BRAF V600E melanoma patient in Cohort 4A (15mg capsule) achieved an unconfirmed partial response with a –31.9% tumor reduction and remains on trial for >11 months; prior best response when treated with a MEK + BRAF combination therapy was stable disease |
| ● | Disease Control Rate (DCR): | |
| ○ | 71.4% (5 of 7) of patients identified with BRAF-mutated tumors achieved stable disease or partial response |
| ○ | 42.8% (9 of 21) of patients achieved stable disease or partial response | |
| ● | Durable Stable Disease: | |
| ○ | A second BRAF V600E melanoma patient previously treated with MEK + BRAF combination therapy in Cohort 6 (30mg capsule) remains on trial for >6 months with a stable disease and tumor shrinkage of -1.6% |
Safety and Tolerability
Among 27 dosed patients through the Dose Limiting Toxicity (DLT) period (Day 28) through the cutoff date of November 10, 2025:
| ● | PAS-004, dosed once daily (QD), has been well-tolerated across all dose levels |
1
| ● | No dose-limiting toxicities (DLTs), and no discontinuations have been reported. | |
| ● | All treatment-related adverse events (TRAEs) at least possible related to PAS-004 were Grade 1 or 2, with limited rash (7.4%), nausea (18.5%), vomiting (14.8%), diarrhea (7.4%), and no ocular retinal abnormalities or cardiovascular toxicities observed. |
Pharmacokinetics (PK)
PAS-004 has demonstrated through Cohort 6:
| ● | Linear PK and dose-proportionality |
| ● | PK curve with Cmax/Cmin ratio <2, with Cmax and Cmin above the IC50 (half-maximal inhibitory concentration) from our cellular assay. |
| ● | Long half-life (~60 hours) |
| ● | Cohort 6 (30mg capsule) has demonstrated: |
| ○ | AUC: ~5,480 ng·h/mL |
| ○ | Cmax: 249 ng/mL |
| ○ | Cmin: 215 ng/mL |
On November 21, 2025, the Company issued a press release (the “November 21 Press Release”) relating to positive tablet PK data from the Company's ongoing Phase 1/1b open-label study evaluating PAS-004 in adult patients with neurofibromatosis type 1 (NF1) with symptomatic and inoperable, incompletely resected, or recurrent plexiform neurofibromas.
Pharmacokinetics (PK)
PAS-004 has demonstrated in the tablet formulation (4mg and 8mg cohorts):
| ● | Linear PK and dose-proportionality |
| ● | PK curve with Cmax/Cmin ratio <2, with Cmax and Cmin above the IC50 (half-maximal inhibitory concentration) from our cellular assay |
| ● | Long half-life (~57 hours) |
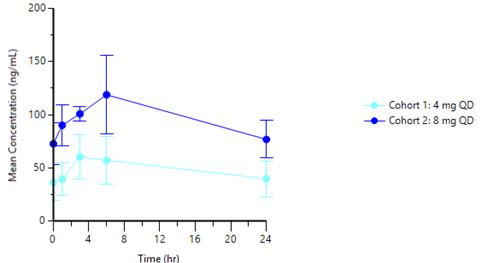
| ● | Cohort 1 (4mg tablet) has demonstrated: |
| ○ | AUC: 1,120 ng·h/mL |
| ○ | Cmax: 58.1 ng/mL |
| ○ | Cmin: 37.6 ng/mL |
| ● | Cohort 2 (8mg tablet) has demonstrated: |
| ○ | AUC: 2,290 ng·h/mL |
| ○ | Cmax: 118 ng/mL |
| ○ | Cmin: 75.4 ng/mL |
2
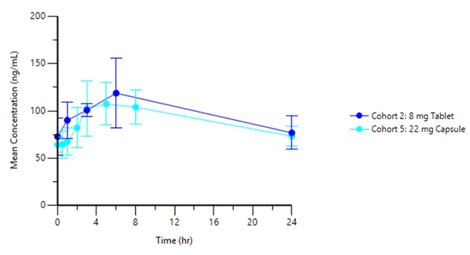
Dose normalized exposures following once daily administration of PAS-004 tablets were approximately 3-fold higher than those following administration with the capsule formulation, resulting in the 8mg tablet area under the curve (AUC) and Cmax being slightly greater than those of the 22mg capsule. The tablet formulation has demonstrated less patient variability and a similar Tmax range when compared to the capsule formulation. This is consistent with the pre-clinical evaluation of the two formulations in the dog toxicology studies.
Graph 1 below represents the tablet PK curve at steady state for the 4mg and 8mg doses and Graph 2 below represents the 8mg tablet PK curve at steady state as compared to 22mg capsule dose at steady state from our ongoing Phase 1 trial in advanced cancer patients:
Graph 1:

Graph 2:
3
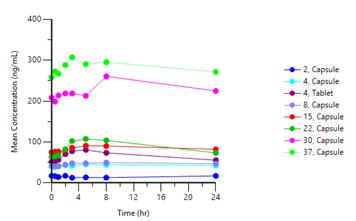
On November 24, 2025, the Company issued a press release (the “November 24 Press Release”) announcing positive safety, PK and PD data from Cohort 7 (37mg capsule) in its ongoing first-in-human trial evaluating PAS-004 in patients with MAPK pathway-driven advanced solid tumors with a documented RAS, NF1 or RAF mutation, or in patients who have failed prior BRAF/MEK inhibition.
PAS-004 has demonstrated in Cohort 7 (37mg capsule):
Safety and Tolerability Results:
| ● | PAS-004 was safe and well tolerated with no dose limiting toxicities (DLTs), and zero treatment-related adverse events observed during the DLT period. |
| ● | After reviewing the safety data, the Safety Review Committee recommended to proceed to Cohort 8, 45mg capsule, without modification. |
Pharmacodynamics (PD) Results:
| ● | At steady-state, patient plasma data showed PAS-004 inhibiting phosphorylated extracellular signal-regulated kinase (pERK) at a level of 80% near Cmax. |
| ● | At steady-state, patient plasma data showed PAS-004 inhibiting pERK at a level above 60% at Cmin (24-hour predose). |
Pharmacokinetics (PK) Results:
| ● | Linear PK and dose-proportionality. |
| ● | PK curve with Cmax/Cmin ratio <2. |
| ● | AUC: 6,690 ng*h/mL; Cmax: 313 ng/mL; Cmin: 260 ng/mL. |
Graph 1 below represents the complete PAS-004 dose escalation curve at steady state in our ongoing Phase 1 trial in advanced cancer patients:
Graph 1:
3
On November 25, 2025, the Company issued a press release (the “November 25 Press Release”) announcing that the ALS Association has awarded a Hoffman ALS Clinical Trial Award grant worth ~$1 million to study PAS-004 in ALS patients. The award was given to study the “Efficacy, safety and tolerability of PAS-004 for the treatment of ALS.”
Forward Looking Statements
This Current Report on Form 8-K contains statements that constitute “forward-looking statements” made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements include statements regarding the Company’s ongoing Phase 1 clinical trial of PAS-004 in advanced cancer patients, the Company’s ongoing Phase 1/1b clinical trial of PAS-004 in adult patients with NF1-associated plexiform neurofibromas, and the safety, tolerability, pharmacokinetic (PK), pharmacodynamics (PD) and preliminary efficacy of PAS-004, as well as all other statements, other than statements of historical fact, regarding the Company’s current views and assumptions with respect to future events regarding its business, as well as other statements with respect to the Company’s plans, assumptions, expectations, beliefs and objectives, the success of the Company’s current and future business strategies, product development, pre-clinical studies, clinical studies, clinical and regulatory timelines, market opportunity, competitive position, business strategies, potential growth and financing opportunities and other statements that are predictive in nature. Forward-looking statements are subject to numerous conditions, many of which are beyond the control of the Company. While the Company believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements, which are based on information available to the Company on the date of this Current Report. These forward-looking statements are based upon current estimates and assumptions and are subject to various risks and uncertainties, including risks that future clinical trial results may not match results observed to date, may be negative or ambiguous, or may not reach the level of statistical significance required for regulatory approval, as well as other factors set forth in the Company’s most recent Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and other filings made with the SEC. Thus, actual results could be materially different. The Company undertakes no obligation to update these statements whether as a result of new information, future events or otherwise, after the date of this Current Report, except as required by law.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| Exhibit No. | Description | |
| 99.1 | Press Release dated November 20, 2025. | |
| 99.2 | Press Release dated November 21, 2025. | |
| 99.3 | Press Release dated November 24, 2025. | |
| 99.4 | Press Release dated November 25, 2025. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document). |
4
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| PASITHEA THERAPEUTICS CORP. | ||
| Date: November 25, 2025 | By: | /s/ Tiago Reis Marques |
| Name: | Tiago Reis Marques | |
| Title: | Chief Executive Officer | |
5