UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
For the fiscal year ended
or
For the transition period from __________________________ to __________________________
Commission file number
(Exact name of registrant as specified in its charter)
| State or other jurisdiction of | (I.R.S. Employer | |
| incorporation or organization | Identification No.) |
| | ||
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including
area code:
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| The | ||||
| Warrants to purchase shares of Common Stock, par value $0.0001 per share | KTTAW | The Nasdaq Capital Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a
well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate by check mark if the registrant is not
required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐
Indicate by check mark whether the registrant
(1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months
(or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements
for the past 90 days.
Indicate by check mark whether the registrant
has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405
of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| Smaller reporting company | |
| Emerging growth company |
If an emerging growth company, indicate by checkmark
if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards
provided pursuant to Section 13(a) of the Exchange Act.
Indicate by check mark whether the registrant
has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial
reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or
issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant
is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐
No
The aggregate market value of the common stock,
par value $0.0001 per share (“Common Stock”), held by non-affiliates of the registrant as of the last business day of the
registrant’s most recently completed second fiscal quarter (June 30, 2022) was $
The registrant had
DOCUMENTS INCORPORATED BY REFERENCE
None.
PASITHEA THERAPEUTICS CORP.
2022 FORM 10-K ANNUAL REPORT
TABLE OF CONTENTS
i
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This annual report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended,. These statements are generally identified by the use of such words as “may,” “could,” “should,” “would,” “believe,” “anticipate,” “forecast,” “estimate,” “expect,” “intend,” “plan,” “continue,” “outlook,” “will,” “potential” and similar statements of a future or forward-looking nature. These forward-looking statements speak only as of the date of filing this annual report with the SEC and include, without limitation, statements about the following:
| ● | our lack of operating history; | |
| ● | the expectation that we will incur significant operating losses for the foreseeable future and will need significant additional capital; | |
| ● | the period over which we estimate our existing cash and cash equivalents will be sufficient to fund our future operating expenses and capital expenditure requirements; | |
| ● | our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; |
| ● | our plans to develop and commercialize our product candidates; |
| ● | the timing of our IND submission for PAS-004; |
| ● | the timing of our planned clinical trials for PAS-004; |
| ● | the ability of our clinical trials to demonstrate safety and efficacy of our future product candidates, and other positive results; | |
| ● | disruptions to the development of our product candidates due to the continued spread of COVID-19 and the resulting global pandemic; |
| ● | the timing and focus of our future preclinical studies and clinical trials, and the reporting of data from those studies and trials; |
| ● | the size of the market opportunity for our future product candidates, including our estimates of the number of patients who suffer from the diseases we are targeting; |
| ● | the success of competing therapies that are or may become available; |
| ● | the beneficial characteristics, safety, efficacy and therapeutic effects of our future product candidates; |
| ● | our ability to obtain and maintain regulatory approval of our future product candidates; |
| ● | our plans relating to the further development of our future product candidates, including additional disease states or indications we may pursue; |
| ● | existing regulations and regulatory developments in the United States and other jurisdictions; | |
| ● | our dependence on third parties; | |
| ● | the need to hire additional personnel and our ability to attract and retain such personnel; |
| ● | our plans and ability to obtain or protect intellectual property rights, including extensions of patent terms where available and our ability to avoid infringing the intellectual property rights of others; |
| ● | our financial performance and sustaining an active trading market for our Common Stock and Warrants; and |
| ● | our ability to restructure our operations to comply with any potential future changes in government regulation. |
Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. You should refer to the “Risk Factors” section of this annual report for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. We operate in an evolving environment and new risk factors and uncertainties may emerge from time to time. It is not possible for management to predict all risk factors and uncertainties. As a result of these factors, we cannot assure you that the forward-looking statements in this annual report will prove to be accurate. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. You should review the factors and risks and other information we describe in the reports we will file from time to time with the SEC.
ii
PART I
ITEM 1. BUSINESS
Overview
We are a biotechnology company primarily focused on the discovery, research and development of innovative treatments for Central Nervous System (“CNS”) disorders and RASopathies. We are leveraging our expertise in the fields of neuroscience, translational medicine, and drug development to advance new molecular entities that target the pathophysiology underlying such diseases, with the goal of bringing life-changing therapies to patients.
We have two business segments, “Therapeutics” and “Clinics.” Our Therapeutics segment performs activities related to discovery, research and development of innovative treatments for CNS disorders and other diseases. Our Clinics segment provided business support services to anti-depression clinics in the U.K. and in the United States. Its operations in the U.K. involved providing business support services to registered healthcare providers who assess patients and, if appropriate, administer intravenous infusions of ketamine. Its operations in the United States involved providing business support services to entities that furnish similar services to patients who personally pay for those services. Operations took place in New York, NY and Los Angeles, CA in the United States, and throughout the U.K. through partnerships with healthcare providers, including Nadelson Medical PLLC and Zen Healthcare. We did not provide professional medical services, psychiatric assessments, or administration of intravenous infusions of ketamine as part of our Clinics segment.
Prior to the date of this Annual Report on Form 10-K, we have discontinued our at-home services in New York, NY as well as our services in the U.K. In addition, we have discontinued our clinical operations in Los Angeles, CA and are actively exploring options for the disposal of related property. Accordingly, as of the date of this Annual Report on Form 10-K, we have discontinued the operations of our Clinics segment.
Our Therapeutic Pipeline
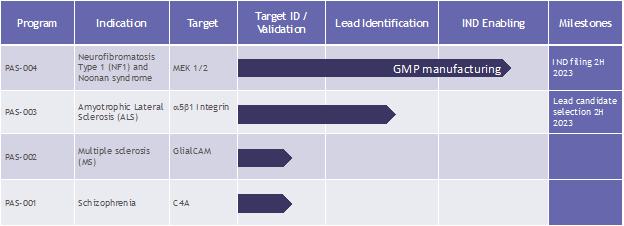
Our therapeutic pipeline currently consists of four programs. Our lead product candidate, PAS-004, is a next-generation macrocyclic (as defined below) mitogen-activated protein kinase, or MEK inhibitor, that we believe may address the limitations and liabilities associated with existing drugs with a similar mechanism of action. Our remaining three programs are in the discovery stage and are based on novel targets that we believe address limitations in the treatment paradigm of the indications we plan to address, which are currently amyotrophic lateral sclerosis (“ALS”), multiple sclerosis (“MS”) and schizophrenia.
1
Our Lead Program: PAS-004
Our lead therapeutic product candidate, PAS-004 (formerly known as “CIP-137401”), is a next-generation MEK 1 and 2 (“MEK 1/2”) inhibitor designed to be macrocyclic for potential use in the treatment of a range of RASopathies, including neurofibromatosis type 1 (“NF1”) and Noonan syndrome, as well as lamin A/C (“LMNA”) cardiomyopathy and a number of oncology indications. We acquired PAS-004 in connection with our acquisition of AlloMek Therapeutics, LLC (“AlloMek”), a privately held biotechnology company, in October 2022.
PAS-004 is a small molecule allosteric inhibitor of MEK 1/2. MEK 1/2 are two of several protein kinases involved in a signaling cascade, known as the mitogen-activated protein kinase, or MAPK pathway. The MAPK pathway is an important pathway in cellular biology which has been a frequent target for drug discovery efforts. The MAPK pathway has been implicated in a variety of diseases, as it functions to drive cell proliferation, differentiation, survival and a variety of other cellular functions that, when abnormally activated, are critical for the formation and progression of tumors, fibrosis and other diseases. MEK inhibitors block phosphorylation (activation) of extracellular signal-regulated kinases (“ERK”). Blocking the phosphorylation of ERK can lead to cell death and inhibition of tumor growth.
PAS-004 has been tested in a range of mouse models of various diseases and has completed preclinical testing and animal toxicology studies to support an Investigational New Drug application (an “IND”) with the U.S. Food and Drug Administration (“FDA”). Additionally, PAS-004 has received orphan-drug designation from the FDA for the treatment of NF1.
Existing FDA approved MEK inhibitors are marketed for a range of diseases, including certain cancers and NF1. We believe these MEK inhibitors suffer from certain limitations, including known toxicities. Unlike current FDA approved MEK inhibitors, PAS-004 is macrocyclic, which we believe may lead to improved pharmacokinetics, tolerability and potency. Macrocycles are large cyclic molecules that can bring increased potency, metabolic stability, and oral bioavailability. Cyclization also offers rigidity for stronger binding with drug target receptors. PAS-004 was designed to provide a longer half-life with what we believe is a better therapeutic window. Further, we believe the potency and safety profile that PAS-004 has demonstrated in preclinical studies may also lead to stronger and more durable response rates and efficacy, as well as better dosing schedules, which may not require the fasting or dietary restrictions of approved MEK inhibitors. However, the ultimate efficacy of PAS-004 cannot be known at this time and until all required clinical testing has been completed.
We plan to submit an IND to the FDA for PAS-004 in the third quarter of 2023, following completion of the ongoing good manufacturing practice (“GMP”) manufacturing of PAS-004 and finalization of our toxicology program. We plan to initially focus our clinical efforts on NF1, potentially followed by Noonan syndrome or other RASopathies, rare diseases with significant unmet clinical needs. Assuming our IND for PAS-004 is accepted by the FDA, we anticipate initiating our first-in-human Phase 1 clinical trial in healthy volunteers as early as possible after our IND is accepted.
Our Discovery Programs
In addition to PAS-004, we are developing our pipeline of discovery programs focused on novel targets for the treatment of CNS disorders that have clear unmet medical needs. Each of our discovery programs is summarized below.
PAS-003
Our PAS-003 program aims to develop a proprietary humanized monoclonal antibody (“mAb”) with a mechanism-of-action targeting a5b1 integrin for the treatment of ALS and other neuroinflammatory disorders, such as MS and possibly stroke. We believe targeting a5b1 integrin may have a beneficial impact on disease due to modulation of multiple cell types and mechanisms involved in neuroinflammation, which occurs in ALS. We acquired PAS-003 in connection with our acquisition of Alpha-5 integrin, LLC, a privately held biotechnology company, in June 2022.
PAS-002
Our PAS-002 discovery program aims to develop a proprietary engineered deoxyribonucleic acid (“DNA”) plasmid tolerizing vaccine targeting GlialCAM (a glial cell adhesion molecule implicated in neurological disease), for the treatment of MS. A published study in Nature in 2022 has shown that GlialCAM, a CNS protein found in the brain’s white matter, is attacked in MS. A component of GlialCAM mimics a component of Epstein-Barr virus (“EBV”) nuclear antigen 1 (“EBNA-1”), which has been shown to likely play a critical role in triggering MS.
2
PAS-001
Our PAS-001 discovery program aims to develop a brain penetrant small molecule targeting the complement component 4A (“C4A”) for the treatment of schizophrenia. Recent findings implicate C4A in synaptic loss (fewer connections between nerve cells), which has been shown to occur in schizophrenia. In humans, greater expression of C4A in the brain is associated with an increased risk of schizophrenia.
Our Strategy
Our mission is to develop innovative therapies to address areas of high unmet medical need, initially in CNS disorders and RASopathies. To achieve our mission, we are executing a near-term strategy with the following key elements:
| ● | Initiate clinical development of PAS-004 for the treatment of NF1. We plan to file an IND with the FDA to study PAS-004 for the treatment of NF1 by the second half of 2023 following completion of GMP manufacturing of PAS-004 and finalization of our toxicology program. Assuming our IND is accepted by the FDA, we anticipate initiating our first-in-human Phase 1 clinical trial in healthy volunteers, as soon as possible after the acceptance of our IND. Assuming we achieve positive safety data in the Phase 1 study, we plan to initiate a Phase 2 proof-of-concept clinical trial in NF1 patients as early as the second half of 2024. |
| ● | Advance PAS-004 into clinical development for Noonan Syndrome or other RASopathies. Based on results from preclinical studies, we believe that PAS-004 may have potential for the treatment of Noonan Syndrome. Following development of PAS-004 for NF1, we may pursue PAS-004 for the treatment of Noonan Syndrome or other RASopathies. As such, we will need to continue testing PAS-004 in relevant preclinical models, develop a formulation of PAS-004 that may be suitable for dosing in infants and the pediatric patient population, and perform additional IND-enabling fetal toxicology studies in non-human primates. We believe we may be eligible for a FDA priority review voucher (“PRV”) for PAS-004 for the treatment of Noonan Syndrome, for which there are currently no FDA approved therapeutics, although somatropin is approved to address the short stature associated with Noonan Syndrome. No assurances can be given that we will receive a PRV for PAS-004. |
| ● | Expand utility of PAS-004 for other indications. Based on results from preclinical studies, we believe that PAS-004 may have potential for the treatment of other diseases, such as LMNA cardiomyopathy and some cancers. We plan to test PAS-004 in various preclinical models to further demonstrate the potential utility of PAS-004 in additional indications. |
| ● | Progress our discovery pipeline to lead candidate selection. We currently plan to continue preclinical development of our discovery programs through lead candidate selection. |
| ● | Maximize the potential of our product candidates with selective use of business development and commercial collaborations. Given our limited resources, following lead candidate selection for each of our discovery programs, we plan to seek business development, non-dilutive funding and collaborative opportunities for continued preclinical and clinical development of our discovery product candidates in order to maximize potential value of each of our discovery programs. We plan to continue evaluating opportunities to work with partners that meaningfully enhance our capabilities with respect to the development and commercialization of our product candidates, which may entail the potential out-licensing of the development and commercialization of our product candidates to larger pharmaceutical organizations, including our lead product candidate PAS-004. Additionally, we plan to establish research collaborations for the continued preclinical and clinical development of our discovery programs. Further, we intend to commercialize our product candidates in key markets either alone or with partners in order to maximize the worldwide commercial potential of our programs. |
3
Overview of Our Lead Program: PAS-004
MAPK Pathway Overview
Signaling pathways describe a series of biological mechanisms in which a group of molecules work together to control a cell function. A cell receives signals from its environment when a molecule binds to a specific receptor on or in the cell. This process may be repeated multiple times through the entire signaling pathway until the last receptor is activated and the cell function is carried out. Abnormal activation of signaling pathways may lead to diseases.
The MAPK pathway, which relies upon the Ras/Raf/MEK/ERK signaling cascade, represents a central biological pathway in all human cells that is responsible for regulating cellular transcription, proliferation and survival. The general structure of the pathway consists of Ras, a small GTPase, and three downstream protein kinases, Raf, MEK and ERK. ERK 1 and 2 (“ERK 1/2”) are structurally similar protein-serine/threonine kinases that regulate a variety of cellular processes including adhesion, migration, survival, differentiation, metabolism, proliferation, transcription, cytoskeletal remodeling and cell cycle progression. MEK 1/2 catalyzes the phosphorylation of ERK 1/2, which is required for enzyme activation. Phosphorylated ERK 1/2 moves to the nucleus, and in turn activates many transcription factors, regulates gene expression, and controls various physiological processes, finally inducing cell repair or cell death.
In addition, at the level of Ras, the pathway is negatively regulated by several proteins, including neurofibromin, the protein encoded by the NF1 gene. Given its direct regulation of ERK, which directly controls downstream signaling through the MAPK pathway, MEK occupies a pivotal position in this signaling cascade and represents a rational small-molecule therapeutic target for multiple diseases, including RASopathies (such as NF1), CNS indications (such as ALS), cardiomyopathies (such as LMNA cardiomyopathy) and oncology indications, where overactivation of the MAPK pathway contributes to disease onset and/or progression.
Background of MEK Inhibitors
MAPK represents one of the most highly targeted signaling pathways in drug development. Several allosteric inhibitors of MEK 1/2 are currently in clinical development. Four of them are approved by the FDA for various oncological indications, with only one approved for NF1. These MEK inhibitors are selective towards MEK 1/2, as they bind to non-ATP-competitive allosteric sites. We believe a limitation of current FDA approved MEK inhibitors are their high rates of serious drug-related adverse events, reported to occur in many treated patients, which may result in drug intolerability. These MEK inhibitors often require increased dosing frequency, which contributes to high rates of adverse events, because the drugs systemically circulate for an extended period of time destroying healthy normal cells, which also rely on the pathway for survival.
Our rationale in developing PAS-004 is to address these shortcomings to potentially provide patients with better outcomes and improved safety.
RASopathies Overview
RASopathies are a clinically defined group of genetic syndromes caused by germline mutations in genes that encode components or regulators of the MAPK pathway. These disorders include neurofibromatosis type 1, Noonan syndrome, capillary malformation–arteriovenous malformation syndrome, Costello syndrome, cardio-facio-cutaneous syndrome, and Legius syndrome. Because of the common underlying MAPK pathway dysregulation amongst all of these syndromes, RASopathies exhibit numerous overlapping phenotypic features, including CNS abnormalities. The MAPK pathway plays an essential role in regulating various cell cycle functions, which are critical to normal human development. MAPK pathway dysregulation has profound deleterious effects on both embryonic and later stages of development, which may cause many of the RASopathies. Therefore, we believe there is a strong scientific rationale for targeting the MAPK pathway with small-molecule therapeutics to treat various RASopathies.
4
Neurofibramtosis-1 (NF-1) Overview
The initial indication we plan to pursue for PAS-004 is the treatment of NF1. NF1 is a RASopathy and part of a group of conditions known as neurocutaneous disorders, conditions that affect the skin and the CNS. NF1 is one of the most common inherited neurological disorders, affecting both children and adults. NF1 affects approximately one in 3,000 newborns throughout the world, with approximately 100,000 patients living in U.S. with NF1. NF1 arises from mutations in the NF1 gene, which encodes for neurofibromin, a key negative regulator of the MAPK pathway.
NF1 is characterized by multiple café au lait (light brown) skin spots and neurofibromas (small benign growths) on or under the skin, and/or freckling in the armpits or groin. Individuals with NF1 may have other manifestations of the disorder, including cardiac malformations, cardiovascular disease, vasculopathy, hypertension, vitamin D deficiency, brain malformations, and seizures. About 50% of people with NF1 also have learning disabilities. Softening and curving of bones, and curvature of the spine (scoliosis) may occur in some patients with NF1. Occasionally, tumors may develop in the brain, on cranial nerves, or on the spinal cord. NF1 is usually diagnosed during childhood. Throughout their lifetime, about 30% to 50% of NF1 patients progress to develop plexiform neurofibromas (“PN”), which are tumors that grow in an infiltrative pattern along the peripheral nerve sheath and can cause severe disfigurement, pain and functional impairment. In some cases NF1-PN may be fatal. NF1-PN are most often diagnosed within the first twenty years of life. These tumors are characterized by aggressive growth, which is typically more rapid during childhood. While NF1-PN are initially benign, these tumors can undergo malignant transformation, leading to malignant peripheral nerve sheath tumors (“MPNST”). NF1 patients have an 8% to 15% lifetime risk of developing MPNST, a diagnosis that carries a 12-month survival rate of under 50%. In addition to MPNST, NF1 patients are at an increased risk of developing other malignancies, including breast cancer and gliomas.
Most patients with NF1-PN are treated with surgical removal of the tumors. However, because NF1-PN arise from nerve cells and grow in an infiltrative pattern, it is challenging to successfully resect tumors without severe comorbidities, such as permanent nerve damage and disfigurement. Patients that are ineligible for surgery or those who have had a recurrence post-surgery are often treated with a variety of off-label therapies. Among these off-label therapies are various systemic treatments, such as chemotherapy and immunotherapy, which have not been shown to consistently confer a clinical benefit. Given that NF1-PN is driven by dysregulation in the MAPK pathway, MEK inhibitors have emerged as a class of therapies that may hold significant promise for the treatment of NF1.
Limitations of Current Standard of Care
Koselugo (selumetinib), a MEK inhibitor, was approved by the FDA in 2020 for NF1 pediatric patients two years of age and older who have symptomatic, inoperable plexiform neurofibromas. Koselugo is the only FDA approved drug for the treatment of NF1. Koselugo is also being evaluated in an ongoing Phase 3 clinical trial for the treatment of adult patients with NF1 who have symptomatic, inoperable PN. In addition to Koselugo, we are aware of several other MEK inhibitors in clinical trials for this indication, as well as the off-label use of other drugs, such as bevacizumab, for the treatment of NF1.
We believe that Koselugo and other earlier generation MEK inhibitors approved for indications other than NFI suffer from limitations, such as an onerous dosing schedule, which requires dosing twice a day on an empty stomach, at least one hour before or two hours after a meal. We believe that this creates a significant market opportunity for a next-generation MEK inhibitor that addresses these shortcomings, has a pharmacokinetic and tolerability profile suitable for long-term dosing and that can arrest or reverse tumor growth.
Noonan Syndrome Overview
The second indication for which we may pursue for PAS-004 is for the treatment of Noonan Syndrome. Noonan Syndrome is a genetic disorder that may be caused by variants in one of several MAPK pathway genes. It affects approximately one in 1,000 to 2,500 newborns. Noonan Syndrome is characterized by distinctive craniofacial features, including a broad forehead, hypertelorism, down-slanting palpebral fissures, and low-set, posteriorly rotated ears. Other features include congenital cardiac defects, reduced growth, bleeding disorders, and a variable degree of neurocognitive delay. Several genes have been shown to be associated with Noonan Syndrome and all these genes encode various components of or proteins associated with the MAPK pathway. Patients with Noonan Syndrome have an increased risk of developing cardiomyopathies as well as several cancers that affect the blood (leukemia), nervous system (neuroblastoma), brain (glioma), muscle (rhabdomyosarcoma), and bones. Patients with Noonan syndrome diagnosed in early childhood with severe hypertrophic cardiomyopathy have an increased mortality risk. Case reports of off label use of MEK inhibitors have shown the potential for prompt clinical improvement and subsequent amelioration of hypertrophic cardiomyopathy as assessed by ultrasound.
5
Limitations of Current Standard of Care
Noonan Syndrome can be diagnosed based on a patient’s medical history and diagnostic tests. There is no approved single treatment for Noonan Syndrome, however management of Noonan syndrome is targeted toward symptomatic improvement and supportive care depending on type and severity. Treatments typically begin around four or five years of age and continue until the child stops growing. There has been evidence of off label use of MEK inhibitors to delay time to a heart transplant in infants diagnosed with Noonan Syndrome.
Preclinical Profile and Mechanism of Action of PAS-004
PAS-004 is a next-generation MEK inhibitor that was rationally designed to have a macrocyclic structure by taking into consideration the metabolic liabilities of earlier generation MEK inhibitors. The structure of PAS-004 is distinct from other earlier generation MEK inhibitors as it maintains critical protein/ligand contacts through sulfonamide and iodophenyl but does not possess a primary alcohol or hydroxamate functionality, a known metabolic liability in earlier generation MEK inhibitors. It is generally observed that macrocyclic scaffolds improve drug-like properties including target binding, selectivity, and oral bioavailability.
PAS-004 has displayed promising pharmacokinetic properties in IND-enabling toxicology studies of both rats and dogs. In these toxicology studies, PAS-004 has demonstrated a half-life of 11.5 hours in rats and 52 hours in dogs. We believe PAS-004’s half-life allows durable suppression of ERK phosphorylation, critical for clinical responses. Further, in these toxicology studies PAS-004 displayed a low peak/trough ratio, which might minimize potential related toxicities.
Preclinical Studies Overview
In vitro Preclinical Studies of PAS-004
In an unpublished preclinical study, the effects of PAS-004 were assessed in the in vivo Colo-205 xenograft tumor model, a common mouse model used for preclinical therapies. Results showed that PAS-004 dosed at 5mg/kg once daily reduced tumor volume. The magnitude of tumor volume reduction was similar to selumetinib dosed at 25mg/kg, twice daily, as published in Molecular Cancer Therapeutics in 2007.
In an unpublished preclinical study, the effects of PAS-004 were compared to selumetinib in human wild type and NF1 deficient Schwann cells, the tumorigenic cell of origin for NF1 plexiform neurofibromas. Preliminary results showed that PAS-004 had minimal activity against wild type cells, however, dose-dependent inhibitory activity against proliferation in NF1 deficient cells was observed.
Additionally, PAS-004 was compared to selumetinib in an in vitro potency assay. Western blots from this unpublished preclinical study showed that cells treated with PAS-004 demonstrated greater reduction in ERK 1/2 phosphorylation as compared to cells treated with selumetinib.
We believe these in vitro preclinical results support PAS-004’s favorable pharmacokinetic profile, potency and dose-dependent inhibitory activity against cellular proliferation in NF1 deficient Schwann cells and appears similar to selumetinib, an FDA approved MEK inhibitor.
In vivo Preclinical Studies
In an unpublished preclinical pilot study, PAS-004 was tested for tolerability and preliminary biological efficacy in a genetically engineered mouse model of NF1 plexiform neurofibromas. These mice were engineered to develop plexiform neurofibromas that closely phenocopy the human tumors by four months of age with 100% penetrance. In this pilot study, selumetinib was administered in a parallel group, which served as a positive control. Both PAS-004 and selumetinib were administered as single-agents to six mice per group. PAS-004 was administered at 10mg/kg once daily and selumetinib was administered at the established maximum tolerated dose of 10mg/kg, twice daily. Treatment began when the mice reached four months of age and was continued for 12 weeks or until death. Mice were monitored for signs of toxicity, as well as survival. Results demonstrated that both PAS-004 and selumetinib showed similar toxicity profiles and both PAS-004 (p=0.0123) and selumetinib (p=0.0048) significantly reduced the tumor size compared to vehicle-treated mice based on statistical analysis using uncorrected Fisher’s least significant difference.
6
We believe the results from this preclinical pilot study show that PAS-004 may be effective in reducing tumor burden of NF1-associated plexiform neurofibromas. When administered at 10mg/kg once daily, PAS-004 and selumetinib, which was dosed at 10mg/kg twice daily, demonstrated similar results. We believe that the longer half-life of PAS-004, as compared to selumetinib, could potentially enhance treatment with superior efficacy by allowing better sustained MEK/ERK signaling inhibition, or allowing for greater intervals between dosing such as single daily dose as compared to the required twice daily dosing for selumetinib.
Mutations in the LMNA gene, which encodes nuclear lamins A and C, cause diseases affecting various organs, including the heart. Studies have found that the ERK 1/2 kinase branches of the MAPK signaling pathway were abnormally hyperactivated prior to the onset of significant cardiac impairment.
PAS-004 was studied in the LMNA-cardiomyopathy LmnaH222P/H222P mouse model, a validated model of cardiomyopathy caused by LMNA mutations in humans. In this study, male mice were orally administered placebo, or PAS-004 at 3 mg/kg/day or PAS-004 at 6 mg/kg/day starting at 14 weeks of age when symptoms of cardiomyopathy were present. Results of this preclinical study were published in Bioorganic & Medicinal Chemistry in 2017 and are summarized as follows:
| ● | The effects of PAS-004 on phosphorylated ERK 1/2 were studied. Following six weeks of systemic administration, both doses of PAS-004 led to significant decreases in phosphorylated ERK 1/2 relative to total ERK 1/2 in the heart and liver when compared to placebo, whereas only the 6 mg/kg/day group produced a significant decrease in phosphorylated ERK 1/2 relative to total ERK 1/2 in quadricep muscles. |
| ● | The effects of PAS-004 on echocardiographic parameters of the heart that correlate with left ventricular function were studied. Following six weeks of systemic administration, both doses of PAS-004 resulted in significant increases in left ventricular fractional shortening, the percentage the left ventricular diameter decreases with each contraction as compared to placebo. |
| ● | The effects of PAS-004 on cardiac fibrosis were studied. Following six weeks of systemic administration, both doses of PAS-004 resulted in significant decreased fibrosis based on staining with Masson trichrome of fixed sections of left ventricles, when compared to placebo. Results showed that treatment of PAS-004 lead to dose-dependent statistically significant decreases in fibrosis as scored on a histologic scale of 0 to 4 by a pathologist blind to treatment group, when compared to placebo. |
| ● | The effects of PAS-004 on survival were studied. Mice followed until death or euthanasia. 23 mice treated with placebo had a median survival of 202 days, whereas median survival was 225 days for 17 mice treated with 3 mg/kg/day of PAS-004 and 225 days for 15 mice treated with 6 mg/kg/day of PAS-004. Results showed the median survivals based on Kaplan-Meier plots of mice treated with both doses of PAS-004 were statistically significantly (P<0.05) longer than that for mice treated with placebo. |
| ● | A preliminary analysis of potential tissue toxicity of PAS-004 was performed. Following six weeks of systemic administration, serum alkaline phosphatase activity, alanine aminotransferase activity and bilirubin concentration were measured to assess possible hepatic injury and liver function. Serum creatinine and blood urea nitrogen concentrations were also measured as indicators of renal function and serum amylase activity as a marker of pancreatic injury. Results showed that there were no statistically significant differences in any of these parameters between groups. A histopathological evaluation by a pathologist blind to treatment determined there were no consistent or specific abnormalities in liver, kidney or spleen of mice receiving either doses of PAS-004 and no alterations were observed that typically occur with drug toxicity. |
7
Toxicology Studies
28-day toxicological studies were performed in both rats and dogs under good laboratory practices (“GLP”) on PAS-004 by Wuxi AppTec (Suzhou) Co., Ltd. We believe the results from these studies have demonstrated a sufficient safety and toxicology profile of PAS-004 to support our IND filing with the FDA. We are conducting an additional 28-day toxicological study in male rats to further support our IND filing with the FDA.
Overview of Our Discovery Programs
PAS-003 Program
Amyotrophic Lateral Sclerosis Overview
ALS, or Lou Gehrig’s disease, is a fatal, progressive motor neuron disease that targets nerve cells in the spinal cord and brain. ALS most commonly affects people between the ages of 40 and 70, with an average age of 55 at the time of diagnosis. It affects as many as 30,000 patients in the United States, with 5,000 new cases diagnosed each year.
While approximately 10% of cases are hereditary, which is known as familial ALS, the large majority of cases (90-95%) are not, which is known as sporadic ALS. A large majority of familial ALS cases are due to genetic mutations in the superoxide dismutase 1 (“SOD1”) gene. While the pathogenesis of ALS is not fully understood, studies have shown that the disease is multifactorial, with several interlinked mechanisms contributing to neurodegeneration, including neuroinflammation, which has been shown to play an important role in neurodegeneration.
ALS often begins with muscle twitching and/or weakness in a limb, however, as the disease progresses, ALS affects control of the muscles needed to move, speak, eat and breathe. As a result, ALS patients develop extensive muscle wasting and atrophy leading to paralysis. The life expectancy is low, with patients living on average three to five years after symptom onset, and the patient´s quality of life is typically poor.
There are currently six FDA approved medications to treat ALS and its symptoms. However, they have been shown to only modestly slow disease progression. Therefore, despite these therapies, the medical need for new treatments for ALS patients is very high.
Scientific Background and Rationale for Targeting a5b1 integrin for the treatment of ALS
Integrins are the principal receptors used by animal cells to bind to the extracellular matrix as well as other cells. Integrins activate intracellular signaling pathways and can cooperate with other conventional signaling receptors. Integrins are involved in a wide range of biological processes including cell growth, migration, survival, and proliferation as well as cytokine activation and release. As a result, integrins play a significant role in many physiological processes, including embryogenesis, organogenesis, and tissue development, but also in pathogenic ones, including inflammation, infection, and allergic and neoplastic diseases.
Integrins are composed by two non-covalently linked alpha and beta subunits. a5b1 integrin, also known as the fibronectin receptor, is a heterodimer consisting of a5 and b1 subunits. Integrins can be broadly grouped based on ligand specificity. In this classification, integrin a5b1 falls under RGD-recognizing integrins and is known to bind fibronectin, osteopontin, fibrillin, thrombospondin, among others. a5b1 integrin has been shown to play a role in cancer, angiogenesis and in a variety of neurological disorders. a5b1 integrin is a validated drug target supported by the clinical development of anti-a5b1 mAbs by several pharmaceutical companies, including PDL Biopharma, Inc. jointly with Biogen Inc., and Pfizer, Inc., for the treatment of cancer indications.
8
In a 2018 Nature Neuroscience publication, scientists at the Steinman Laboratory at Stanford University, headed by our Chairman, Prof. Lawrence Steinman, used mass cytometry to identify an upregulation of CD49e (a5 integrin) on brain myeloid cells in the mutant SOD1-G93A mouse model of ALS and demonstrated that a5b1 integrin is upregulated on microglia in the CNS as the disease progresses. Additional preclinical studies have shown that a5 integrin is also elevated on macrophages in the periphery and suggest a role for mast cells which also express high level of a5 integrin.
In collaboration with the Mayo Clinic, we have shown a5b1 integrin positive endothelial cells are concentrated in post-mortem human brain motor neuron tracts but not in sensory regions in ALS and that a5b1 expression increases with disease progression in both mouse models of ALS and human ALS patients. Previous studies have shown that high levels of a5b1 integrin on the endothelium plays a role in angiogenesis while our results suggest a role in the blood brain barrier which regulates immune cell trafficking.
Together these findings indicate that a5b1 expression increases with disease progression in mouse models of ALS and in human ALS patients and highlight the role of a5 b1 integrin on four different cell types involved in neuroinflammation in ALS: microglia, macrophages, mast cells and endothelial cells. We believe these findings suggest that targeting a5b1 integrin may provide a treatment for ALS. In this regard, initial results from the Steinman Laboratory and our own preclinical studies have demonstrated that anti-a5b1 treatment improved motor function and increased survival in SOD transgenic mice, the most phenotypically relevant preclinical model for ALS.
We believe these preclinical results demonstrate that targeting a5b1 integrin has the potential to be a powerful new therapy that could improve ALS outcomes. Our goal is to select a lead product candidate for our PAS-003 discovery program in the second half of 2023 and seek partnerships and/or collaborators to continue development of the program.
PAS-002
Multiple Sclerosis Overview
MS is an auto-immune chronic inflammatory demyelinating disease affecting the CNS. According to the National MS Society, there are more than 2.8 million people worldwide with a diagnosis of MS. In the United States a recently completed prevalence study, funded by the National MS Society, estimated that nearly one million people over the age of 18 live with a diagnosis of MS.
Most people with MS have a relapsing-remitting disease course. They experience periods of new symptoms or relapses that develop over days or weeks and usually improve partially or completely. These relapses are followed by quiet periods of disease remission that can last months or even years. Approximately two-thirds of those with relapsing-remitting MS can eventually develop a steady progression of symptoms, with or without periods of remission, within 10 to 20 years from disease onset. This is known as secondary-progressive MS. Approximately 10% of people with MS experience a gradual onset and steady progression of signs and symptoms without any relapses, known as primary-progressive MS.
The exact cause of MS is unknown, but changes in the peripheral immune system and intrinsic CNS immune cells (such as microglia) contribute to MS pathogenesis. Acute and chronic inflammation as well as neurodegeneration occur throughout the disease course, with prominence of acute inflammation in the relapsing phase of disease. Both innate and adaptive immune responses play a role in MS. The adaptive immune response includes CD8+ cytotoxic T cells as well as CD4+ T cells, in particular Th1 cells, against myelin proteins. Furthermore, B cells also play a role by mean of antigen presentation to T cells, antibody formation and production of proinflammatory cytokines. MS lesions (focal areas of myelin damage) ultimately causes the symptoms of MS.
Recent studies have proved that Epstein-Barr virus (“EBV”), triggers MS by priming the immune system to attack the body’s own nervous system. A 2022 study published in Science analyzed EBV antibodies in serum from 801 individuals who developed MS among a cohort of more than10 million people active in the U.S. military over a 20-year period. This study showed that EBV infection was present in all but one case at the time of MS onset, and found that of 35 people who were initially EBV-negative, all but one became infected with EBV before the onset of MS. This finding provides compelling data implicating EBV as the trigger for the development of MS.
9
Scientific Background and Rationale for Targeting GLIALCAM for the treatment of MS
GlialCAM found in the brain’s white matter is attacked in MS. GlialCAM is a CNS protein that has a component that mimics a component of EBNA-1, which plays a critical role in triggering MS. This study elucidated the molecular mimicry between EBNA-1 and GlialCAM, and GlialCAM’s role in the pathogenesis of MS. It also demonstrated that targeting the adaptive immune response to EBNA-1 and GlialCAM with approaches aimed at tolerization of the autoimmune response and eradication of the EBV infection in the B lymphocyte lineage. These findings demonstrate a mechanistic link between EBV infection and the pathobiology of MS and create new pathways for the clinical treatment of multiple sclerosis.
In an initial preclinical proof-of-concept study in a mouse model of relapsing-remitting experimental autoimmune encephalomyelitis (“EAE”), the standard animal model of MS, we showed that an engineered DNA tolerizing vaccine targeting GlialCAM reduced disease severity and incidence of relapse when administered prophylactically in the EAE model. Based on these results, we are continuing to study engineered DNA plasmids in additional proof-of-concept studies and plan to publish results when complete.
In addition, we are investigating different lipid nanoparticle delivery systems for delivery of the DNA plasmid.
We believe these early results in EAE models demonstrate that developing a DNA plasmid tolerizing vaccine targeting GlialCAM has the potential to reduce disease severity and incidence in relapsing-remitting MS and possibly result in a long-term cure. Our goal is to develop the PAS-002 to lead candidate selection and seek partnerships and/or collaborators to continue development of the program.
PAS-001
Schizophrenia Overview
Schizophrenia is a chronic and disabling psychiatric illness characterized by positive psychotic symptoms, such as delusions and hallucinations, negative symptoms, such as social withdrawal and amotivation, and impairment in cognitive domains, including attention, working memory, verbal learning and executive function. According to the World Health Organization (“WHO”) schizophrenia affects approximately 24 million people, or one in 300 people worldwide. Schizophrenia has a low lifetime prevalence of about 1%, however the burden of the disease is substantial. Schizophrenia is a leading cause of adult disease burden and has been ranked 12th in the top global causes of disability for the last decade, leading to substantial healthcare and societal costs, with annual associated costs in the U.S. estimated to be more than $150 billion.
Current pharmacological treatments for schizophrenia all act on dopamine D2 receptors. Although they are effective in reducing positive symptoms, they have little effect on both cognitive and negative symptoms. Furthermore, up to 30% of patients show only partial benefit with antipsychotics and have treatment resistant schizophrenia. This highlights the need for new therapeutic strategies.
Despite extensive research the molecular etiology remains unknown. The current dopamine hypothesis postulates that excessive striatal dopamine transmission and reduced frontal dopamine stimulation underlie the pathophysiology of positive and negative symptoms, respectively. However, converging lines of genetic, epidemiological and clinical evidence indicate that inflammatory pathways are also altered in schizophrenia. More recently, a leading hypothesis proposes that synaptic terminal loss is central to the pathophysiology of schizophrenia, leading to impaired cortical function, and symptoms, including cognitive impairments.
Scientific Background and Rationale for Targeting C4A for the treatment of Schizophrenia
The complement system is a group of proteins found in blood plasma and on some cell surfaces. These proteins play an important role in protecting against infection and removing dead cells and foreign material. In the brain, the complement system plays a crucial role in immune response and in synaptic elimination during normal development and disease. There are nine major complement proteins, labeled C1 through C9. Complement protein C4 is the only complement protein that has two different isotypes encoded by two different genes: C4A and C4B.
10
Microglia are phagocytes residing in the CNS. Unlike other phagocytes, which primarily function in immunity, microglia are heavily involved in shaping and supporting brain tissue. Microglia use immune molecules, such as complement proteins, to send signals to neurons and glia, and to survey their microenvironment using dynamic processes. Microglia are key modulators of neuronal development. However, the full role they play in healthy brain development and disease remain elusive.
In studies, C4A has been shown to mark synapses for phagocytosis by microglia. Further, the C4 gene has been linked to synaptic refinement and psychiatric disorders, including schizophrenia. In humans, greater expression of C4A in the brain is associated with an increased risk of schizophrenia.
The largest genome-wide association study (GWAS) in schizophrenia in 2014 identified 128 independent associations spanning 108 conservatively defined loci that meet genome-wide significance, including the major histocompatibility complex (MHC) locus on chromosome 6, which includes the C4 gene, thus furthering the hypothesis of C4 as an important genetic risk factor in schizophrenia. These results established a link between complement-mediated synaptic pruning and dendritic spine loss in the cortex of schizophrenic patients.
Animal models of increased C4 expression show reduced levels of synaptic proteins and increased phagocytosis of synaptic terminals by microglia. Moreover, preclinical models show C4 overexpression leads to reduced neurotransmission in prefrontal cortical neurons, reduced social interaction and impaired memory, which mimic similar abnormalities seen in schizophrenia patients. Finally, excessive microglial synapse elimination has been observed in schizophrenia-derived in vitro models. Post-mortem brain analyses showed that C4 is expressed at significantly higher levels in people with schizophrenia than controls. C4 levels in cerebro-spinal fluid (“CSF”) have shown to be elevated in patients with schizophrenia relative to matched controls and correlates with CSF measurements of synapse density. C4 levels have also been found to be elevated in plasma in schizophrenia, and higher levels predict poorer outcomes in first episode patients.
Several other studies in scientific journals, including a 1997 study from Psychiatry Research, a 2016 study from Nature and a 2012 study from Revista Brasileira de Psiquiatria, have also reported increased complement gene expression, protein concentration, and overall activity in the serum or plasma of schizophrenia cases compared to controls. Further, a 2020 study published in Brain, Behavior and Immunity, found that C4 was overexpressed in the dorsolateral prefrontal cortex, parietal cortex, superior temporal gyrus and associative striatum of patients with schizophrenia and that C4 expression was not altered in the peripheral tissues of schizophrenia patients. Further, the study found lifelong C4 overexpression in the brain of schizophrenia patients. Taken together, this evidence has led to the hypothesis that schizophrenia is a neuroimmune disorder mediated by alterations in pro- and anti-inflammatory processes in the CNS.
We are currently developing a brain-penetrant small molecule able to down regulate C4A, a novel neuroinflammatory pathway, for the systemic treatment of schizophrenia. The initial development work and screening is currently being conducted by Evotec, utilizing Evotec’s integrated research and development expertise and state-of-the-art structure-based drug design techniques. Our goal is to continue screening and early development of PAS-001 and seek partnerships and/or collaborators to continue further preclinical development of the program.
Recent Acquisitions
Alpha-5 Integrin Therapeutics, LLC
On June 21, 2022, we entered into a Membership Interest Purchase Agreement (the “Alpha-5 Agreement”) with PD Joint Holdings, LLC Series 2016-A and Prof. Lawrence Steinman (the “Alpha-5 Sellers”), pursuant to which we purchased from the Alpha-5 Sellers all of the issued and outstanding equity of Alpha-5 integrin, LLC, a Delaware limited liability (“Alpha-5”). The Alpha-5 Sellers were the sole title and beneficial owners of 100% of the equity interests of Alpha-5. In consideration of the equity of Alpha-5, the Alpha-5 Sellers received (i) an aggregate of 3,260,870 shares (the “Alpha-5 Shares”) of our Common Stock, (ii) warrants to purchase 1,000,000 shares of our Common Stock at an exercise price of $1.88 per share (the “Alpha-5 Warrants”), and (iii) contingent earn-out payments of an aggregate of 2% to 4% of net sales generated from the sale of a drug currently in development by Alpha-5.
11
Prof. Lawrence Steinman, one of the Alpha-5 Sellers, is our Executive Chairman and Co-Founder of the Company, and as such is considered a related party. The terms of the Alpha-5 Agreement were approved by (i) the disinterested members of the audit committee (“Audit Committee”) of our board of directors (the “Board”) and (ii) the disinterested members the Board, under the Company’s related party transaction policy.
In connection with the Alpha-5 Agreement, each of the employees of Alpha-5 entered into employment agreements with the Company.
AlloMek Therapeutics, LLC
On October 11, 2022, we entered into a Membership Interest Purchase Agreement, dated October 11, 2022 (the “AlloMek Agreement”), by and among the Company, AlloMek Therapeutics, LLC, a Delaware limited liability company (the “AlloMek”), the persons listed on Schedule 1.1 thereto (each individually a “AlloMek Seller” and collectively, “Sellers”), and Uday Khire, not individually but in his capacity as the representative of Sellers (the “AlloMek Representative”), pursuant to which we purchased all of the issued and outstanding equity of AlloMek. The AlloMek Sellers were the sole title and beneficial owners of 100% of the equity interests of AlloMek. In consideration of the sale of the equity of AlloMek, the AlloMek Sellers received (i) an aggregate of 2,700,000 shares of our Common Stock, (ii) warrants to purchase an aggregate of 1,000,000 shares of our Common Stock (the “AlloMek Warrants”) at an exercise price of $1.88 per share, which may be exercised on a cashless basis, for a period of five years commencing on the date of issuance, (iii) a cash payment in the amount of $1.05 million, (iv) the right to certain milestone payments in an amount up to $5.0 million, and (v) the right to contingent earn-out payments ranging from 3% to 5% of net sales of the Drug (as defined in the AlloMek Agreement) depending on the amount of such net sales in the applicable measurement period.
12
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly evolving technologies, intense competition, and an emphasis on proprietary product candidates. While we believe that our technology, development experience and scientific knowledge provide us with competitive advantages, we face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical, and biotechnology companies, academic institutions, governmental agencies and public and private research institutions. Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future.
Many of our competitors may have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals, and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. Moreover, potential competitors have or may have patents or other rights that conflict with patents covering our technologies.
The key competitive factors affecting the success of all our product candidates, if approved, are likely to be their efficacy, safety, side effects, convenience, price, the level of generic competition, and the availability of reimbursement from government and other third-party payors.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient, or are less expensive than any product candidates that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. In addition, our ability to compete may be affected in many cases by insurers or other third-party payors seeking to encourage the use of generic products.
PAS-004
Companies with FDA approved MEK inhibitors include: GSK plc, which received FDA approval for Mekinist (trametinib), which was subsequently sold to Novartis AG; Pfizer Inc., which received FDA approval for Mektovi (binimetinib); Genentech, Inc., a member of the Roche Company, which received FDA approval for Cotellic (cobimetinib); and AstraZeneca and Merck & Co., Inc., which received FDA approval for Koselugo (selumetinib). There are other MEK inhibitors in various stages of clinical trials for multiple indications, including various cancers and NF1. Additionally, there are other FDA approved small molecule therapeutics that target the MAPK signaling pathway.
13
Intellectual Property
Our ability to obtain, maintain and enforce intellectual property protection for our products candidates, formulations, processes, methods and any other proprietary technologies, preserve our trade secrets, and operate without infringing on the proprietary rights of other parties, both in the United States and in other countries is fundamental to the long-term success of our business. Our policy is to actively seek to obtain, where appropriate, the broadest intellectual property protection possible for our current product candidates and any future product candidates, proprietary information and proprietary technology through a combination contractual arrangements and patents, both in the United States and abroad. However, patent protection may not afford us with complete protection against competitors who seek to circumvent our patents.
We also depend upon the skills, knowledge, experience and know-how of our management and research and development personnel, as well as that of our advisors, consultants and other contractors. To help protect our proprietary know-how, which is not patentable, and for inventions for which patents may be difficult to enforce, we currently rely and will in the future rely on trade secret protection and confidentiality agreements to protect our interests. To this end, we require all of our employees, consultants, advisors and other contractors to enter into confidentiality agreements that prohibit the disclosure of confidential information and, where applicable, require invention assignment agreements to us of the ideas, developments, discoveries and inventions important to our business.
We generally control access to our proprietary and confidential information through the use of internal controls that are subject to periodic review. Although we take steps to protect our proprietary information and trade secrets, third parties may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or disclose our technology. As a result, we may not be able to meaningfully protect our trade secrets. For further discussion of the risks relating to intellectual property, see the section titled “Risk Factors—Risks Related to Our Intellectual Property.”
Our patent portfolio includes issued and pending applications worldwide for each of our programs.
PAS-004
For PAS-004, we have issued patents titled “Novel MEK inhibitors, useful in the treatment of diseases” that have claims directed to composition of matter and methods of use, and includes granted patents in the United States, Australia, Canada, China, Germany, Spain, France, Italy, Great Britain, India and Japan, that are expected to expire in October of 2030 (without consideration of patent term adjustment (“PTA”) and patent term extension (“PTE”)).
PAS-003
For PAS-003, we have pending patent applications in three patent families. The first patent family has claims directed to monoclonal antibodies. The second patent family has claims directed to humanized monoclonal antibodies. The third patent family has claims directed to methods of treating stroke. Patents that may issue worldwide in these families will have a statutory expiration date in May of 2042 to November 2043 (without consideration of PTA and PTE).
PAS-002
For PAS-002, we have pending patent applications in two patent families that are directed to GlialCAM tolerizing therapies. Patents that may issue worldwide in these families will have a statutory expiration date in 2043 (without consideration of PTA and PTE).
Grant Agreements
FightMND Grant
In connection with the acquisition of Alpha-5, we legally assumed rights under a three-year grant agreement with FightMND, a not-for-profit Australian charity, which was entered into by Alpha-5 on September 23, 2021. FightMND supports preclinical research, development and assessment of therapeutics for Motor Neuron Disease/Amyotrophic Sclerosis. Under the grant agreement, we are entitled to reimbursements for costs incurred up to $967,010 AUD for research related to a monoclonal antibody targeting a5b1 integrin as a potential treatment for ALS.
14
Manufacturing
We contract with third parties for the manufacture of our product candidates for preclinical studies and clinical trials, and we intend to continue to do so in the future. For PAS-004, we currently work with one contract manufacturing organization (“CMO”), WuXi STA, a subsidiary of WuXi AppTec (“Wuxi”) for the manufacture of PAS-004 drug substance and plan to utilize Wuxi for the manufacture of drug product for our clinical trials. We do not own or operate, and currently have no plans to establish, any manufacturing facilities. We utilize an outside CMC consultant with pharmaceutical development and manufacturing experience who are responsible for the relationships with our CMO.
We believe that the use of contract CMOs eliminates the need to directly invest in manufacturing facilities, equipment and additional staff. Although we rely on contract manufacturers, our personnel and consultants have extensive manufacturing experience overseeing CMCs and CMOs.
As we further develop our product candidates, we expect to consider secondary or back-up manufacturers for both active pharmaceutical ingredient and drug product manufacturing. To date, our CMO has met the manufacturing requirements for our product candidates in a timely manner. We expect third-party manufacturers to be capable of providing sufficient quantities of our product candidates to meet our current needs, but we have not assessed these capabilities beyond the supply of clinical materials to date. We currently engage CMOs on a ‘‘fee for services’’ basis based on our current development plans.
Employees & Human Capital
As of December 31, 2022, we had 15 full time employees, of which seven employees were related to our Therapeutics segment and eight employees were related to our Clinics segment. None of our employees are represented by a labor union or covered by a collective bargaining agreement.
We believe that our future success will depend, in part, on our continued ability to attract, hire and retain qualified personnel. In particular, we depend on the skills, experience and performance of our senior management and research personnel. We compete for qualified personnel with other medical pharmaceutical, and healthcare companies, as well as universities and non-profit research institutions.
We provide competitive compensation and benefits programs to help meet the needs of our employees. In addition to salaries, these programs (which vary by country/region and employment classification) include incentive compensation plans, healthcare and insurance benefits, retirement investments, paid time off, and family leave, among others. We also use targeted equity-based grants with vesting conditions to facilitate retention of personnel, particularly for our key employees.
The success of our business is fundamentally connected to the well-being of our people. Accordingly, we are committed to the health and safety of our employees. In response to the COVID-19 pandemic, we implemented significant changes that we determined were in the best interest of our employees, as well as the communities in which we operate, and which comply with government regulations.
We consider our relations with our employees to be good.
Facilities
Our principal executive office is located at 1111 Lincoln Road, Suite 500, Miami Beach, FL 33139. We rent approximately 300 square feet of space, which includes our executive offices. Our research and development facility is located at 458 Carlton Court, South San Francisco, CA. We rent approximately 1,900 square feet of space, which includes our laboratory and offices.
Website
Our website is www.pasithea.com. On our website, investors can obtain, free of charge, a copy of our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, our Code of Conduct and Business Ethics, including disclosure related to any amendments or waivers thereto, other reports and any amendments thereto filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act of 1934, as amended, as soon as reasonably practicable after we file such material electronically with, or furnish it to, the Securities and Exchange Commission, or the SEC. None of the information posted on our website is incorporated by reference into this Annual Report. The SEC also maintains a website at http://www.sec.gov that contains reports, proxy and information statements and other information regarding us and other companies that file materials with the SEC electronically.
Government Regulation and Drug Approval
Government authorities in the United States (including federal, state and local authorities) and in other countries, extensively regulate, among other things, the manufacturing, research and clinical development, marketing, labeling and packaging, storage, distribution, post-approval monitoring and reporting, advertising and promotion, pricing and export and import of pharmaceutical products, such as our future product candidates. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Moreover, failure to comply with applicable regulatory requirements may result in, among other things, warning letters, clinical holds, civil or criminal penalties, recall or seizure of products, injunction, disbarment, partial or total suspension of production or withdrawal of the product from the market. Any agency or judicial enforcement action could have a material adverse effect on us.
15
U.S. Government Regulation
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act (“FDCA”) and its implementing regulations. Drugs are also subject to other federal, state and local statutes and regulations. The FDA’s Center for Drug Evaluation and Research would have primary jurisdiction over the premarket development, review and approval of our future product candidates. Accordingly, we have and plan to continue to investigate our products through the IND framework and seek approval through the NDA and BLA pathways. The process required by the FDA before our product candidates may be marketed in the United States generally involves the following:
| ● | submission to the FDA of an IND which must become effective before human clinical trials may begin and must be updated annually; | |
| ● | completion of extensive preclinical laboratory tests and preclinical animal studies, all performed in accordance with the FDA’s Good Laboratory Practice regulations; | |
| ● | performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the product candidate for each proposed indication in accordance with good clinical practice (“GCP”); | |
| ● | submission to the FDA of an NDA or BLA after completion of all pivotal clinical trials; | |
| ● | a determination by the FDA within 60 days of its receipt of an NDA or BLA to file the NDA or BLA for review; | |
| ● | satisfactory completion of an FDA pre-approval inspection of the manufacturing facilities at which the active pharmaceutical ingredient (“API”), and finished drug product are produced and tested to assess compliance with good manufacturing Practices (“cGMP”) regulations; and | |
| ● | FDA review and approval of an NDA or BLA prior to any commercial marketing or sale of the drug in the United States. |
An IND is a request for authorization from the FDA to administer an investigational drug product to humans. The central focus of an IND submission is on the general investigational plan and the protocol(s) for human studies. The IND also includes results of animal studies or other human studies, as appropriate, as well as manufacturing information, analytical data and any available clinical data or literature to support the use of the investigational new drug. An IND must become effective before human clinical trials may begin. An IND will automatically become effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to the proposed clinical trials. In such a case, the IND may be placed on clinical hold and the IND sponsor and the FDA must resolve any outstanding concerns or questions before clinical trials can begin. Accordingly, submission of an IND may or may not result in the FDA allowing clinical trials to commence.
Clinical trials involve the administration of the investigational drug to human subjects under the supervision of qualified investigators in accordance with GCP, which include the requirement that all research subjects provide their informed consent for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety, and the efficacy criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. Additionally, approval must also be obtained from each clinical trial site’s institutional review board (“IRB”) before the trials may be initiated, and the IRB must monitor the study until completed. There are also requirements governing the reporting of ongoing clinical trials and clinical trial results to public registries.
16
The clinical investigation of a drug or biologic is generally divided into three phases. Although the phases are usually conducted sequentially, they may overlap or be combined. The three phases of an investigation are as follows:
| ● | Phase I. Phase I includes the initial introduction of an investigational new drug into humans. Phase I clinical trials are typically closely monitored and may be conducted in patients with the target disease or condition or in healthy volunteers. These studies are designed to evaluate the safety, dosage tolerance, metabolism and pharmacologic actions of the investigational drug in humans, the side effects associated with increasing doses, and if possible, to gain early evidence on effectiveness. During Phase I clinical trials, sufficient information about the investigational drug’s pharmacokinetics and pharmacological effects may be obtained to permit the design of well-controlled and scientifically valid Phase II clinical trials. The total number of participants included in Phase I clinical trials varies, but is generally in the range of 20 to 80. |
| ● | Phase II. Phase II includes controlled clinical trials conducted to preliminarily or further evaluate the effectiveness of the investigational drug for a particular indication(s) in patients with the disease or condition under study, to determine dosage tolerance and optimal dosage, and to identify possible adverse side effects and safety risks associated with the drug. Phase II clinical trials are typically well-controlled, closely monitored, and conducted in a limited patient population, usually involving no more than several hundred participants. |
| ● | Phase III. Phase III clinical trials are generally controlled clinical trials conducted in an expanded patient population generally at geographically dispersed clinical trial sites. They are performed after preliminary evidence suggesting effectiveness of the drug has been obtained, and are intended to further evaluate dosage, clinical effectiveness and safety, to establish the overall benefit-risk relationship of the investigational drug product, and to provide an adequate basis for product approval. Phase III clinical trials usually involve several hundred to several thousand participants. |
A pivotal study is a clinical study which adequately meets regulatory agency requirements for the evaluation of a drug candidate’s efficacy and safety such that it can be used to justify the approval of the product. Generally, pivotal studies are also Phase III studies but may be Phase II studies if the trial design provides a well-controlled and reliable assessment of clinical benefit, particularly in situations where there is an unmet medical need.
The FDA, the IRB or the clinical trial sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk. Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group provides authorization for whether or not a trial may move forward at designated check points based on access to certain data from the study. We may also suspend or terminate a clinical trial based on evolving business objectives and/or competitive climate.
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, detailed investigational drug product information is submitted to the FDA in the form of an NDA or BLA requesting approval to market the product for one or more indications. The application includes all relevant data available from pertinent preclinical and clinical trials, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product’s chemistry, manufacturing, controls and proposed labeling, among other things. Data can come from company-sponsored clinical trials intended to test the safety and effectiveness of a use of a product, or from a number of alternative sources, including studies initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and effectiveness of the investigational drug product to the satisfaction of the FDA.
17
Once the NDA or BLA submission has been accepted for filing, within 60 days following submission, the FDA’s goal is to review applications for new molecular entities within ten months of the filing date or, if the application relates to a serious or life-threatening indication and demonstrates the potential to provide a significant improvement in safety or effectiveness over currently marketed therapies, six months from the filing date. The review process is often significantly extended by FDA requests for additional information or clarification. The FDA may refer the application to an advisory committee for review, evaluation and recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it typically follows such recommendations.
After the FDA evaluates the NDA or BLA and conducts inspections of manufacturing facilities where the drug product and/or its active pharmaceutical ingredient will be produced, it may issue an approval letter or a complete response letter. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. A complete response letter indicates that the review cycle of the application is complete and the application is not ready for approval. A complete response letter may require additional clinical data and/or an additional pivotal Phase III clinical trial(s), and/or other significant, expensive and time-consuming requirements related to clinical trials, preclinical studies or manufacturing. Even if such additional information is submitted, the FDA may ultimately decide that the NDA or BLA does not satisfy the criteria for approval. The FDA could also approve the NDA or BLA with a risk evaluation and mitigation strategy (REMS) to mitigate risks, which could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. The FDA also may condition approval on, among other things, changes to proposed labeling, development of adequate controls and specifications, or a commitment to conduct one or more post-market studies or clinical trials. Such post-market testing may include Phase IV clinical trials and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization. Regulatory approval of oncology products often requires that patients in clinical trials be followed for long periods to determine the overall survival benefit of the drug.
After regulatory approval of a drug product is obtained, manufacturers are required to comply with a number of post-approval requirements. The holder of an approved NDA or BLA must report, among other things, certain adverse reactions and production problems to the FDA, to provide updated safety and efficacy information, and to comply with requirements concerning advertising and promotional labeling for the approved product. Also, quality control and manufacturing procedures must continue to conform to cGMP after approval to ensure and preserve the long-term stability of the drug product. The FDA periodically inspects manufacturing facilities to assess compliance with cGMP, which imposes extensive procedural, substantive and record keeping requirements. In addition, changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon us and any third-party manufacturers that we may decide to use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance.
We expect to rely on third parties for the production of clinical and commercial quantities of our future product candidates. Future FDA and state inspections may identify compliance issues at our facilities or at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct. In addition, discovery of previously unknown problems with a product or the failure to comply with applicable requirements may result in restrictions on a product, manufacturer or holder of an approved NDA or BLA, including withdrawal or recall of the product from the market or other voluntary, FDA-initiated or judicial action that could delay or prohibit further marketing. Newly discovered or developed safety or effectiveness data may require changes to a product’s approved labeling, including the addition of new warnings and contraindications, and also may require the implementation of other risk management measures. Also, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our products under development.
18
Expedited Development and Review Programs for Drugs
The FDA maintains several programs intended to facilitate and expedite development and review of new drugs and biologics to address unmet medical needs in the treatment of serious or life-threatening diseases or conditions. These programs include Fast Track designation, Breakthrough Therapy designation, Priority Review and Accelerated Approval, and the purpose of these programs is to either expedite the development or review of important new drugs to get them to patients more quickly than standard FDA review timelines typically permit.
A drug is eligible for Fast Track designation if it is intended to treat a serious or life-threatening disease or condition and demonstrates the potential to address unmet medical needs for such disease or condition. Fast Track designation provides increased opportunities for sponsor interactions with the FDA during preclinical and clinical development, in addition to the potential for rolling review once a marketing application is filed. Rolling review means that the agency may review portions of the marketing application before the sponsor submits the complete application. In addition, a drug may be eligible for Breakthrough Therapy designation if it is intended to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Breakthrough Therapy designation provides all the features of Fast Track designation in addition to intensive guidance on an efficient drug development program, and FDA organizational commitment to expedited development, including involvement of senior managers and experienced review staff in a cross-disciplinary review, where appropriate.
Any product submitted to the FDA for approval, including a product with Fast Track or Breakthrough Therapy designation, may also be eligible for additional FDA programs intended to expedite the review and approval process, including Priority Review designation and Accelerated Approval. A product is eligible for Priority Review designation, once an NDA or a biologics license application, or BLA, is submitted, if the drug that is the subject of the marketing application has the potential to provide a significant improvement in safety or effectiveness in the treatment, diagnosis or prevention of a serious disease or condition. Under priority review, the FDA’s goal date to take action on the marketing application is six months compared to ten months for a standard review. Products are eligible for Accelerated Approval if they can be shown to have an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or an effect on an intermediate clinical endpoint that can be measured earlier than an effect on irreversible morbidity or mortality, which is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments.
Accelerated Approval is usually contingent on a sponsor’s agreement to conduct additional post-approval studies to verify and describe the product’s clinical benefit. The FDA may withdraw approval of a drug or an indication approved under Accelerated Approval if, for example, the confirmatory trial fails to verify the predicted clinical benefit of the product. In addition, the FDA generally requires, as a condition for Accelerated Approval, that all advertising and promotional materials intended for dissemination or publication within 120 days of marketing approval be submitted to the agency for review during the pre-approval review period. After the 120-day period has passed, all advertising and promotional materials must be submitted at least 30 days prior to the intended time of initial dissemination or publication.
Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or the time period for FDA review or approval may not be shortened. Furthermore, Fast Track designation, Breakthrough Therapy designation, Priority Review and Accelerated Approval do not change the scientific or medical standards for approval or the quality of evidence necessary to support approval, though they may expedite the development or review process.
The Rare Pediatric Disease Designation and Priority Review Voucher Program
Under the FD&C Act, the FDA incentivizes the development of drugs and biologics that meet the definition of a “rare pediatric disease,” defined to mean a serious or life-threatening disease in which the serious or life-threatening manifestations primarily affect individuals aged from birth to 18 years and the disease affects fewer than 200,000 individuals in the United States or affects more than 200,000 in the United States and for which there is no reasonable expectation that the cost of developing and making such product for such disease or condition will be received from sales in the United States. To be eligible for the incentives, a sponsor must first request and receive from FDA, prior to or with an NDA or BLA submission, a rare pediatric disease designation. The FDA must deem the application eligible for priority review (i.e., the product treats a serious condition and, if approved, would provide a significant improvement in safety or effectiveness). If the rare pediatric product is approved, the sponsor may be eligible for a voucher that can be used to obtain a priority review for a subsequent, different NDA or BLA for any use, pediatric or not. Rare pediatric disease designation does not guarantee that a sponsor will receive a priority review voucher (PRV) upon approval of its NDA or BLA. If a PRV is received, it may be sold or transferred an unlimited number of times. Under current law, PRVs can be granted only for products that receive rare disease designation by September 30, 2024, and that are approved by September 30, 2026.
19
U.S. Patent Term Restoration
Depending upon the timing, duration, and specifics of the FDA approval of the use of our current and potential product candidates, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984 (“Hatch-Waxman Amendments”). The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of an NDA or BLA plus the time between the submission date of a BLA or NDA and the approval of that application. Only one patent applicable to an approved biological product is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent. The U.S. Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration.
Disclosure of Clinical Trial Information
Sponsors of clinical trials of FDA-regulated drugs and biologics are required to register and disclose certain clinical trial information on the website www.clinicaltrials.gov. Information related to the product, patient population, phase of investigation, trial sites and investigators, and other aspects of a clinical trial are then made public as part of the registration. Sponsors are also obligated to disclose the results of their clinical trials after completion. Disclosure of the results of clinical trials can be delayed in certain circumstances for up to two years after the date of completion of the trial. Competitors may use this publicly available information to gain knowledge regarding the progress of clinical development programs as well as clinical trial design.
Pediatric Information
Under the Pediatric Research Equity Act (“PREA”), NDAs and BLAs must contain data to assess the safety and effectiveness of the product for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA may grant full or partial waivers, or deferrals, for submission of data. Unless otherwise required by regulation, PREA does not apply to any product with orphan product designation except a product with a new active ingredient that is a molecularly targeted cancer product intended for the treatment of an adult cancer and directed at a molecular target determined by FDA to be substantially relevant to the growth or progression of a pediatric cancer that is subject to an NDA or BLA submitted on or after August 18, 2020.
The Best Pharmaceuticals for Children Act (“BPCA”) provides a six-month extension of any non-patent exclusivity for a drug or biologic if certain conditions are met. Conditions for exclusivity include the FDA’s determination that information relating to the use of a new drug or biologic in the pediatric population may produce health benefits in that population, the FDA making a written request for pediatric studies, and the applicant agreeing to perform, and reporting on, the requested studies within the statutory timeframe. Applications under the BPCA are treated as priority applications, with all of the benefits that designation confers.
Controlled Substances
The federal Controlled Substances Act of 1970, or CSA, and its implementing regulations establish a “closed system” of regulations for controlled substances. The CSA imposes registration, security, recordkeeping and reporting, storage, manufacturing, distribution, importation and other requirements under the oversight of the DEA. The DEA is the federal agency responsible for regulating controlled substances, and requires those individuals or entities that manufacture, import, export, distribute, research, or dispense controlled substances to comply with the regulatory requirements in order to prevent the diversion of controlled substances to illicit channels of commerce.
20
The DEA categorizes controlled substances into one of five schedules - Schedule I, II, III, IV or V - with varying qualifications for listing in each schedule. Schedule I substances by definition have a high potential for abuse, have no currently accepted medical use in treatment in the United States and lack accepted safety for use under medical supervision. Pharmaceutical products having a currently accepted medical use that are otherwise approved for marketing may be listed as Schedule II, III, IV or V substances, with Schedule II substances presenting the highest potential for abuse and physical or psychological dependence, and Schedule V substances presenting the lowest relative potential for abuse and dependence.
Facilities that manufacture, distribute, import or export any controlled substance must register annually with the DEA. The DEA registration is specific to the particular location, activity(ies) and controlled substance schedule(s).
The DEA, and some states, also conduct periodic inspections of registered establishments that handle controlled substances. Facilities that conduct research, manufacture, store, distribute, import or export controlled substances must be registered to perform these activities and have the security, control and inventory mechanisms required by the DEA to prevent drug loss and diversion. Failure to maintain compliance, particularly non-compliance resulting in loss or diversion, can result in regulatory action that could have a material adverse effect on our business, results of operations, financial condition and prospects. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to revoke those registrations. In certain circumstances, violations could lead to criminal proceedings.
The states also maintain separate controlled substance laws and regulations, including licensing, recordkeeping, security, distribution, and dispensing requirements. State authorities, including boards of pharmacy, regulate use of controlled substances in each state. Failure to maintain compliance with applicable requirements, particularly as manifested in the loss or diversion of controlled substances, can result in enforcement action that could have a material adverse effect on our business, operations and financial condition. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to revoke those registrations. In certain circumstances, violations could lead to criminal prosecution.
Europe/Rest of World Government Regulation
In addition to regulations in the United States, we may be subject to a variety of regulations in other jurisdictions governing, among other things, clinical trials and any commercial sales and distribution of our future product candidates.
Whether or not we obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in foreign countries prior to the commencement of clinical trials or marketing of the product in those countries. Certain countries outside of the United States have a similar process that requires the submission of a clinical trial application much like the IND prior to the commencement of human clinical trials. In Europe, for example, a clinical trial application (“CTA”), must be submitted to each country’s national health authority and an independent ethics committee, much like the FDA and IRB, respectively. Once the CTA is approved in accordance with a country’s requirements, clinical trial development may proceed.
Following the U.K.’s exit from the European Union, a separate regulatory regime applies in the U.K. to clinical trials and licensing of medicines.
The requirements and process governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. In all cases, the clinical trials are conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
To obtain regulatory approval of an investigational drug under EU regulatory systems, we must submit a marketing authorization application. The EMA is responsible for the scientific evaluation of centralized MAA. Once granted by the European Commission, the centralized marketing authorization is valid in all EU Member States, Iceland, Norway and Liechtenstein. The application used to file the NDA or BLA in the United States is similar to that required in Europe, with the exception of, among other things, country-specific document requirements.
21
For other countries outside of the EU, such as countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. In all cases, again, the clinical trials are conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Authorization Procedures in the European Union
In all cases, the application for marketing approval requires the completion of clinical trials. Clinical trials are currently regulated under Directive 2001/20/EC. EU directives are not directly applicable in the Member States. They have to be transposed into national law. National law transposing EU directives often varies to a great extent. However, in April 2014 a new regulation on clinical trials on medicinal products for human use was adopted. Regulations are directly applicable in the Member States, so they generally lead to greater harmonization. Regulation 536/2014 (“CTR”), entered into force on in June 2014. The CTR will harmonize the assessment and supervision processes for clinical trials throughout the EU via a Clinical Trials Information System, or CTIS, which will contain a centralized EU portal and database for clinical trials. The exact timing of the Regulation’s application depends on confirmation of full functionality of CTIS through an independent audit.
Medicines can be authorized in the EU by using either the centralized authorization procedure or national authorization procedures.
| ● | Centralized Procedure (regulated in Regulation (EC) 726/2004). Under the Centralized Procedure a so-called Community Marketing Authorization is issued by the European Commission, based on the opinion of the Committee for Medicinal Products for Human Use of the European Medicines Agency (“EMA”). The Community Marketing Authorization is valid throughout the entire territory of the European Economic Area (“EEA”) (which includes the 27 Member States of the EU plus Norway, Liechtenstein and Iceland). The Centralized Procedure is mandatory for certain types of products, such as biotechnology medicinal products, orphan medicinal products, and medicinal products indicated for the treatment of AIDS, cancer, neurodegenerative disorders, diabetes, auto-immune and viral diseases. The Centralized Procedure is optional for products containing a new active substance not yet authorized in the EEA, or for products that constitute a significant therapeutic, scientific or technical innovation or which are in the interest of public health in the EU. For medicines that do not fall within these categories, an applicant has the option of submitting an application for a centralized marketing authorization to the EMA, as long as the medicine concerned is a significant therapeutic, scientific or technical innovation, or if its authorization would be in the interest of public health. |
| ● | Cooperative Authorization Procedures (regulated in Directive 2001/83/EC and implemented into Member States’ national law). There are also two other possible routes to authorize medicinal products in several countries, which are available for investigational drug products that fall outside the scope of the centralized procedure: |
| ● | Decentralized Procedure. Using the Decentralized Procedure, an applicant may apply for simultaneous authorization in more than one EU country of medicinal products that have not yet been authorized in any EU country and that do not fall within the mandatory scope of the centralized procedure. Under the Decentralized Procedure the applicant chooses one country as Reference Member State. The regulatory authority of the Reference Member State will then be in charge of leading the assessment of the marketing authorization application. |
| ● | Mutual Recognition Procedure. In the Mutual Recognition Procedure, a medicine is first authorized in one EU Member State, in accordance with the national procedures of that country. Following this, further marketing authorizations can be sought from other EU countries in a procedure whereby the countries concerned agree to recognize the validity of the original, national marketing authorization. |
| ● | Furthermore, there is the option to obtain a national authorization in just one Member State. |
22
In the EU, upon receiving marketing authorization, new chemical entities generally receive eight years of data exclusivity and an additional two years of market exclusivity. If granted, data exclusivity prevents regulatory authorities in the EU from referencing the innovator’s data to assess a generic application. During the additional two-year period of market exclusivity, a generic marketing authorization can be submitted, and the innovator’s data may be referenced, but no generic product can be marketed until the expiration of the market exclusivity. However, there is no guarantee that a product will be considered by the EU’s regulatory authorities to be a new chemical entity, and there is a risk that products may not qualify for data exclusivity.
U.K. Regulation
The Medicines and Healthcare products Regulatory Agency (MHRA) is an executive agency of the Department of Health and Social Care in the U.K. which is responsible for ensuring that medicines and medical devices work and are acceptably safe.
The MHRA has the following roles:
| ● | Operate post-marketing surveillance - in particular the Yellow Card Scheme - for reporting, investigating and monitoring of adverse drug reactions to medicines and incidents with medical devices. | |
| ● | Assess and authorize medicinal products for sale and supply in the U.K. | |
| ● | Oversee the Notified Bodies that ensure medical device manufacturers comply with regulatory requirements before putting devices on the market. | |
| ● | Operate a quality surveillance system to sample and test medicines to address quality defects and to monitor the safety and quality of unlicensed products. | |
| ● | Investigate internet sales and potential counterfeiting of medicines, and prosecute where necessary. | |
| ● | Regulate clinical trials of medicines and medical devices. | |
| ● | Monitor and ensure compliance with statutory obligations relating to medicines and medical devices. | |
| ● | Promote safe use of medicines and devices. |
In the United Kingdom and following the United Kingdom’s exit from the European Union, EU medicines regulation has been adopted as standalone United Kingdom legislation with some amendments to reflect procedural and other requirements with respect to marketing authorizations and other regulatory provisions.
In order to market a medicinal product in the United Kingdom, a license or marketing authorization must be obtained from the MHRA The United Kingdom legislation includes multiple assessment routes for applications for medicinal products, including a 150-day national assessment or a rolling review application. Further, and for a transitional period until 31 December 2022, the MHRA may rely on a decision taken by the European Commission on the approval of a new marketing authorization in the centralized procedure. In addition, the MHRA has the power to have regard to marketing authorizations approved in EU member states.
The United Kingdom has adopted new legislation, the Medicines and Medical Devices Act 2021 and may make changes to the licensing or authorization of medicines in the future. The separate UK authorization system, albeit with transitional recognition procedures in the UK, may lead to additional regulatory costs. In addition, further regulatory costs will be incurred with respect to the lack of mutual recognition of batch testing and related regulatory measures between the European Union and the United Kingdom.
The CQC is an executive non-departmental public body of the Department of Health and Social Care of the U.K. It regulates and inspects health and social care services in England and registration is required prior to the provision of health and care services. Further, certain drug and pharmaceutical licenses and registrations may be required for the possession and/or supply of certain drugs.
The GPhC is the body responsible for the independent regulation of the pharmacy profession within Great Britain (England, Scotland and Wales) regulation and enforcement by, responsible for the regulation of pharmacists, pharmacy technicians and pharmacy premises.
Zen Healthcare has established consultants and advisors to ensure it operates in accordance with the CQC. Zen Healthcare also has responsibility under our agreements to obtain all the regulatory approvals and licenses to operate from the aforementioned bodies and complies with the MHRA, CQC and GPhC.
23
Other Health Care Laws
We may also be subject to healthcare regulation and enforcement by the US federal government and the states and foreign governments where we may market our product candidates, if approved. The US laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, physician sunshine and privacy and security laws and regulations with corresponding laws in non-US countries.
The US federal Anti-Kickback Statute prohibits, among other things, any person from knowingly and willfully offering, soliciting, receiving or providing remuneration, directly or indirectly, to induce either the referral of an individual, for an item or service or the purchasing or ordering of a good or service, for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs. The Anti-Kickback Statute is subject to evolving interpretations. In the past, the government has enforced the Anti-Kickback Statute to reach large settlements with healthcare companies based on sham consulting and other financial arrangements with physicians. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act. The majority of states also have anti-kickback laws which establish similar prohibitions and, in some cases, may apply to items or services reimbursed by any third-party payor, including commercial insurers.
Additionally, the US Civil False Claims Act prohibits knowingly presenting or causing the presentation of a false, fictitious or fraudulent claim for payment to the United States government. Actions under the False Claims Act may be brought by the Attorney General or as a qui tam action by a private individual in the name of the government. Violations of the False Claims Act can result in very significant monetary penalties and treble damages. The federal government is using the False Claims Act, and the accompanying threat of significant liability, in its investigation and prosecution of pharmaceutical and biotechnology companies throughout the United States, for example, in connection with the promotion of products for unapproved uses and other sales and marketing practices. The government has obtained multi-million and multi-billion-dollar settlements under the False Claims Act in addition to individual criminal convictions under applicable criminal statutes. Given the significant size of actual and potential settlements, it is expected that the government will continue to devote substantial resources to investigating healthcare providers’ and manufacturers’ compliance with applicable fraud and abuse laws.
HIPAA also created new federal criminal statutes that prohibit among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
There has also been a recent trend of increased federal and state regulation of payments made to physicians and other healthcare providers. The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, (collectively, “the Affordable Care Act”), among other things, imposed new reporting requirements on drug manufacturers for payments made by them to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Failure to submit timely, accurately and completely the required information may result in civil monetary penalties of up to an aggregate of approximately $0.2 million per year (or up to an aggregate of $1.2 million per year for “knowing failures”), for all payments, transfers of value or ownership or investment interests that are not timely, accurately and completely reported in an annual submission. Drug manufacturers are required to submit reports to the government by the 90th day of each calendar year. Certain states also mandate implementation of compliance programs, impose restrictions on drug manufacturer marketing practices and/or require the tracking and reporting of marketing expenditures and pricing information as well as gifts, compensation and other remuneration to physicians.
24
We may also be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. HIPAA, as amended by HITECH, and their respective implementing regulations, including the final omnibus rule published on January 25, 2013, imposes specified requirements relating to the privacy, security and transmission of individually identifiable health information. Among other things, HITECH makes HIPAA’s privacy and security standards directly applicable to “business associates,” defined as independent contractors or agents of covered entities that create, receive, maintain or transmit protected health information in connection with providing a service for or on behalf of a covered entity. HITECH also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce HIPAA and seek attorney’s fees and costs associated with pursuing such civil actions. In addition, state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways, thus complicating compliance efforts.
Coverage and Reimbursement
Sales of our product candidates, once approved, will depend, in part, on the extent to which the costs of our products will be covered by third-party payors, such as government health programs, private health insurers and managed care organizations. Third-party payors generally decide which drugs they will cover and establish certain reimbursement levels for such drugs. In particular, in the United States, private health insurers and other third-party payors often provide reimbursement for products and services based on the level at which the government (through the Medicare or Medicaid programs) provides reimbursement for such treatments. Patients who are prescribed treatments for their conditions and providers performing the prescribed services generally rely on third-party payors to reimburse all or part of the associated healthcare costs. Patients are unlikely to use our products unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our products. Sales of our products and product candidates, if approved, will therefore depend substantially on the extent to which the costs of products and our product candidates will be paid by third-party payors. Additionally, the market for our products and future product candidates will depend significantly on access to third-party payors’ formularies without prior authorization, step therapy, or other limitations such as approved lists of treatments for which third-party payors provide coverage and reimbursement. Additionally, coverage and reimbursement for therapeutic products can differ significantly from payor to payor. One third-party payor’s decision to cover a particular medical product or service does not ensure that other payors will also provide coverage for the medical product or service, or will provide coverage at an adequate reimbursement rate. As a result, the coverage determination process will require us to provide scientific and clinical support for the use of our products to each payor separately and will be a time-consuming process.
In addition, the United States government, state legislatures and foreign governments have continued implementing cost-containment programs, including price controls, restrictions on coverage and reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our future net revenue and results. Decreases in third-party reimbursement for our products and future product candidates or a decision by a third-party payor to not cover our products or future product candidates could reduce physician usage of our products and future product candidates, if approved, and have a material adverse effect on our sales, results of operations and financial condition.
Health Care Reform
In the United States and foreign jurisdictions, there have been a number of legislative and regulatory changes to the healthcare system that could affect our future results of operations. There have been and continue to be a number of initiatives at the United States federal and state levels that seek to reduce healthcare costs.
In particular, in the United States, the Affordable Care Act has had, and is expected to continue to have, a significant impact on the healthcare industry. The Affordable Care Act was designed to expand coverage for the uninsured while at the same time containing overall healthcare costs. The Affordable Care Act, among other things, addressed a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected, increased the minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program and extended the rebate program to individuals enrolled in Medicaid managed care organizations, established annual fees and taxes on manufacturers of certain branded prescription drugs, and established a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts, which, through subsequent legislative amendments, was increased to 70%, off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D. Substantial new provisions affecting compliance were also enacted, which may require us to modify our business practices with healthcare providers and entities.
25
Since its enactment, there have been judicial and Congressional challenges to certain aspects of the Affordable Care Act. If a law is enacted, many if not all of the provisions of the ACA may no longer apply to prescription drugs. While we are unable to predict what changes may ultimately be enacted, to the extent that future changes affect how any future products are paid for and reimbursed by government and private payers our business could be adversely impacted. In November 2020, Joseph Biden was elected President and, in January 2021, the Democratic Party obtained control of the Senate. As a result of these electoral developments, it is unlikely that continued legislative efforts will be pursued to repeal ACA. Instead, it is possible that legislation will be pursued to enhance or reform ACA. We are not able to state with certainty what the impact of potential legislation will be on our business.
In addition, other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. Recently there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed bills designed to, among other things, reform government program reimbursement methodologies. Individual states in the United States have also become increasingly active in implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our future product candidates or additional pricing pressures.
Facilities and Operational Regulation
U.S.
Federal, state and local regulations (implemented by CMS, FDA, the Occupational Health and Safety Administration (“OSHA”), the DEA, and state departments or boards of public health, public welfare, medicine, nursing, pharmacy, and medical assistance, among others) would require us to meet various standards relating to, among other things, the management, licensing, safety, security and operation of facilities (including, e.g., laboratories, pharmacies, and clinics), personnel qualifications and licensing, the maintenance of proper records, equipment, and quality assurance programs, and the dispensing, storage, and administration of controlled substances. All of our clinics and facilities in the U.S. would be subject to periodic inspection by federal, state and local agencies to determine if the operations, premises, equipment, personnel and patient care meet applicable standards.
Our operations are subject to various federal, state and local hazardous and medical waste disposal laws. As currently in effect, laws governing the disposal of hazardous waste do not classify most of the waste produced in connection with the provision of our health care services as hazardous, although disposal of non-hazardous medical waste is subject to specific state regulation. Our operations are also subject to various air emission and wastewater discharge regulations.
Non-U.S.
We would be subject to a broad spectrum of regulation in other countries. Our operations must comply with various environmental and transportation regulations in the countries in which we operate. Our facilities and clinics are also subject to various standards relating to, among other things, facilities, management, personnel qualifications and licensing, maintenance of proper records, equipment, quality assurance programs, the operation of pharmacies, the protection of workers from blood-borne diseases and the dispensing of controlled substances. All of our operations may be subject to periodic inspection by various governmental authorities to determine if the operations, premises, equipment, personnel and patient care meet applicable standards. Our clinic operations and our related activities generally require licenses, which may be subject to periodic renewal and may be revoked for violation of applicable regulatory requirements.
In addition, many countries impose various investment restrictions on foreign companies. For instance, government approval may be required to enter into a joint venture with a local partner. Some countries do not permit foreign investors to own a majority interest in local companies or require that companies organized under their laws have at least one local stockholder. Investment restrictions therefore affect the corporate structure, operating procedures and other characteristics of our subsidiaries and joint ventures in these and other countries.
26
ITEM 1A. RISK FACTORS
Our future operating results could differ materially from the results described in this annual report due to the risks and uncertainties described below. You should consider carefully the following information about risks in evaluating our business. If any of the following risks actually occur, our business, financial condition, results of operations and future growth prospects would likely be materially and adversely affected. Additional risks and uncertainties not presently known to us or that we currently deem immaterial also may impair our business operations in these circumstances, the market price of our securities would likely decline. In addition, we cannot assure investors that our assumptions and expectations will prove to be correct. Important factors could cause our actual results to differ materially from those indicated or implied by forward-looking statements. See “Forward Looking Statements” for a discussion of some of the forward-looking statements that are qualified by these risk factors. Factors that could cause or contribute to such differences include those factors discussed below.
Summary Risk Factors
The following summarizes key risks and uncertainties that could materially adversely affect us. You should read this summary together with the more detailed description of each risk factor contained below.
| ● | We are a clinical stage biopharmaceutical company with a limited operating history. | |
| ● | We have incurred a history of operating losses and expect to continue to incur substantial costs for the foreseeable future. We are not currently profitable, and we may never achieve or sustain profitability. | |
| ● | We will need to raise additional capital to complete the development and commercialization efforts for PAS-004 and our other product candidates. If we are unable to raise capital when needed, we could be forced to delay, reduce or terminate certain of our development programs or other operations. | |
| ● | A pandemic, epidemic, or outbreak of an infectious disease, such as COVID-19, could cause a disruption to the development of our product candidates. | |
| ● | We are dependent primarily on the successful development and commercialization of our lead product candidate, PAS-004, which is not yet approved. Our business could be materially adversely affected if one or more of our key product candidates do not perform as well as expected and do not receive regulatory approval. We cannot give any assurance that we will receive regulatory approval for such product candidate or any other product candidates which is necessary before any of our product candidates can be commercialized. | |
| ● | Even if we obtain regulatory approval for PAS-004, or any of our other product candidates, such approval may be limited, and we will be subject to stringent, ongoing government regulation The commercial success of our product candidates, if approved, depends partially upon attaining market acceptance by physicians, patients, third-party payors, and the medical community. | |
| ● | Our business is subject to extensive regulatory requirements, and our product candidates that obtain approval will be subject to ongoing and continued regulatory review, which may result in significant expense and limit our ability to commercialize such products. | |
| ● | We expect to rely on third parties to conduct our clinical trials and our regulatory submissions for our product candidates, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials and/or regulatory submissions. | |
| ● | We may rely on third parties to perform many essential services for any products that we commercialize, including distribution, customer service, accounts receivable management, cash collection and adverse event reporting. If these third parties fail to perform as expected or to comply with legal and regulatory requirements, our ability to commercialize PAS-004 or our other product candidates will be significantly impacted and we may be subject to regulatory sanctions. | |
| ● | We will need to further increase the size and complexity of our organization in the future, and we may experience difficulties in executing our growth strategy and managing any growth. | |
| ● | Our research and development is focused on discovering and developing product candidates which may not make it to the market. | |
| ● | We are increasingly dependent on information technology, and our systems and infrastructure face certain risks, including cybersecurity and data leakage risks. | |
| ● | If our intellectual property related to our products or product candidates is not adequate, we may not be able to compete effectively in our market. | |
| ● | An active trading market for our Common Stock or warrants to purchase shares of our Common Stock that were issued in our Initial Public Offering and are listed on Nasdaq (the “Warrants”) may not be sustained. |
27
Risks Related to Our Financial Position and Need for Additional Capital
We have a limited operating history and have no products or services approved for commercial sale, which may make it difficult for you to evaluate our current business and predict our future success and viability.
We have a limited operating history upon which you can evaluate our business and prospects. We have no products or services approved for commercial sale and have not generated any material revenue from product sales. To date, we have devoted substantially all of our resources and efforts to organizing and staffing our company, business planning, and product candidate development. We have not yet demonstrated our ability to obtain marketing approvals, manufacture a commercial-scale product or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. As a result, it may be more difficult for you to accurately predict our future success or viability than it could be if we had a longer operating history.
Accordingly, you should consider our prospects in light of the costs, uncertainties, delays and difficulties frequently encountered by companies in the early stages of development, especially preclinical stage pharmaceutical companies such as ours. Potential investors should carefully consider the risks and uncertainties that a company with a limited operating history will face. In particular, potential investors should consider that we cannot assure you that we will be able to, among other things:
| ● | successfully implement or execute our current business plan, and we cannot assure you that our business plan is sound; |
| ● | successfully manufacture our clinical product candidates and establish commercial supply; |
| ● | successfully complete the clinical trials necessary to obtain regulatory approval for the marketing of our product candidates; |
| ● | secure market exclusivity and/or adequate intellectual property protection for our product candidates; |
| ● | attract and retain an experienced management and advisory team; |
| ● | secure acceptance of our product candidates in the medical community and with third-party payors and consumers; |
| ● | raise sufficient funds in the capital markets or otherwise to effectuate our business plan; and |
| ● | utilize the funds that we do have and/or raise in the future to efficiently execute our business strategy. |
If we cannot successfully execute any one of the foregoing, our business may fail and your investment will be adversely affected.
We have a history of losses and may not be able to achieve profitability going forward.
We are a preclinical stage biopharmaceutical company with a limited operating history and have incurred losses since our formation. We incurred net losses of approximately $13.9 million and $2.2 million for the years ended December 31, 2022 and 2021, respectively. As of December 31, 2022, we had an accumulated deficit of approximately $18.7 million. We have not commercialized any product candidates and have never generated revenue from the commercialization of any product. To date, we have devoted most of our financial resources to research and development, including our preclinical work, general and administrative expenses, as well as to intellectual property.
We expect to incur significant additional operating losses for the next several years, at least, as we advance our product candidates through preclinical development, complete clinical trials, seek regulatory approval and commercialization, if any our product candidates are approved. The costs of advancing product candidates into each clinical phase tend to increase substantially over the duration of the clinical development process. Therefore, the total costs to advance any of our product candidates to marketing approval in even a single jurisdiction will be substantial. Because of the numerous risks and uncertainties associated with pharmaceutical product development, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to begin generating revenue from the commercialization of any products or achieve or maintain profitability. Our expenses will also increase substantially if and as we:
| ● | establish a sales, marketing and distribution infrastructure to commercialize our drugs, if approved, and for any other product candidates for which we may obtain marketing approval; |
| ● | maintain, expand and protect our intellectual property portfolio; |
| ● | hire additional clinical, scientific and commercial personnel; |
| ● | add operational, financial and management information systems and personnel, including personnel to support our product development and planned future commercialization efforts, as well as to support our transition to a public reporting company; and |
| ● | acquire or in-license or invent other product candidates or technologies. |
28
Furthermore, our ability to successfully develop, commercialize and license any product candidates and generate product revenue is subject to substantial additional risks and uncertainties, as described under “Risks Related to Development, Clinical Testing, Manufacturing, Regulatory Approval and Commercialization.” As a result, we expect to continue to incur net losses and negative cash flows for the foreseeable future. These net losses and negative cash flows have had, and will continue to have, an adverse effect on our stockholders’ equity and working capital. The amount of our future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenues. If we are unable to develop and commercialize one or more product candidates, either alone or through collaborations, or if revenues from any product that receives marketing approval are insufficient, we will not achieve profitability. Even if we do achieve profitability, we may not be able to sustain profitability or meet outside expectations for our profitability. If we are unable to achieve or sustain profitability or to meet outside expectations for our profitability, the value of our Common Stock and Warrants will be materially and adversely affected.
We will require additional capital to fund our operations, and if we fail to obtain necessary financing, we may not be able to complete the development and commercialization of our drugs.
Our operations have consumed substantial amounts of cash since inception. We expect to continue to spend substantial amounts to advance the clinical development of and launch and commercialize our product candidates if we receive regulatory approval. We will require additional capital for the further development and potential commercialization of our product candidates and may also need to raise additional funds sooner to pursue a more accelerated development of our product candidates, if available to us. If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, reduce or eliminate our research and development programs or any future commercialization efforts.
At December 31, 2022, we had cash of approximately $33.1 million. We have incurred continuing losses including a loss of $13.9 million for the year ended December 31, 2022. Our future funding requirements, both near and long-term, will depend on many factors, including, but not limited to the:
| ● | initiation, progress, timing, costs and results of preclinical studies and clinical trials, including patient enrollment in such trials, for our product candidates or any other future product candidates; |
| ● | clinical development plans we establish for our product candidates and any other future product candidates; |
| ● | obligation to make royalty and non-royalty sublicense receipt payments to third-party licensors, if any, under our licensing agreements; |
| ● | number and characteristics of product candidates that we discover or in-license and develop; |
| ● | outcome, timing and cost of regulatory review by the FDA and comparable foreign regulatory authorities, including the potential for the FDA or comparable foreign regulatory authorities to require that we perform more studies than those that we currently expect; |
| ● | costs of filing, prosecuting, defending and enforcing any patent claims and maintaining and enforcing other intellectual property rights; |
| ● | effects of competing technological and market developments; |
| ● | costs and timing of the implementation of commercial-scale manufacturing activities; |
| ● | costs and timing of establishing sales, marketing and distribution capabilities for any product candidates for which we may receive regulatory approval; |
| ● | cost associated with being a public company. |
If we are unable to expand our operations or otherwise capitalize on our business opportunities due to a lack of capital, our ability to become profitable will be compromised.
29
Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial revenue, we may finance our cash needs through a combination of equity offerings, debt financings, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements or other sources. We do not currently have any committed external source of funds. In addition, we may seek additional capital due to favorable market conditions or strategic considerations, even if we believe that we have sufficient funds for our current or future operating plans.
To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a common stockholder. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may be required to relinquish valuable rights to our technologies, intellectual property, future revenue streams or product candidates or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate product candidate development or future commercialization efforts.
Changes in U.S. tax law may materially adversely affect our financial condition, results of operations and cash flows.
On March 27, 2020, the Coronavirus Aid, Relief, and Economic Security Act, or the CARES Act, was signed into law to address the COVID-19 crisis. The CARES Act is an approximately $2 trillion emergency economic stimulus package that includes numerous U.S. federal income tax provisions, including the modification of: (i) net operating loss rules (as discussed below), (ii) the alternative minimum tax refund and (iii) business interest deduction limitations under Section 163(j) of the Internal Revenue Code of 1986, as amended, or the Code.
On December 22, 2017, President Trump signed into law federal tax legislation commonly referred to as the TCJA (defined below), which also significantly changed the U.S. federal income taxation of U.S. corporations. TCJA remains unclear in many respects and has been, and may continue to be, subject to amendments and technical corrections, as well as interpretations and implementing regulations by the Treasury and Internal Revenue Service, or the IRS, any of which could lessen or increase certain adverse impacts of TCJA. In addition, it is unclear how these U.S. federal income tax changes will affect state and local taxation, which often uses federal taxable income as a starting point for computing state and local tax liabilities.
The Tax Cuts and Jobs Act (TJCA) (P.L. 115-97) modified the section 174 rules and beginning in 2022, taxpayers may no longer currently deduct R&D expenditures but instead must amortize specified R&D expenditures ratably over five years (or 15 years for foreign expenditures).
While some of these U.S. federal income tax changes may adversely affect us in one or more reporting periods and prospectively, other changes may be beneficial on a going-forward basis. We continue to work with our tax advisors and auditors to determine the full impact TCJA and the CARES Act will have on us. We urge our investors to consult with their legal and tax advisors with respect to both TCJA and the CARES Act and the potential tax consequences of investing in our Common Stock and Warrants.
Our ability to use our net operating losses and other tax attributes may be limited.
As of December 31, 2022, we had approximately $14.5 million of federal and $7.3 million of state net operating loss carryforwards (“NOLs”), available to offset future taxable income. Under Sections 382 and 383 of the U.S. Internal Revenue Code of 1986, as amended, or the Code, a corporation that undergoes an “ownership change,” generally defined as a greater than 50% change by value in its equity ownership over a three-year period is subject to limitations on its ability to utilize its pre-change NOLs and other tax attributes such as research tax credits to offset future taxable income. We have not performed an analysis to determine whether our past issuances of stock and other changes in our stock ownership may have resulted in other ownership changes. If it is determined that we have in the past experienced other ownership changes, or if we undergo one or more ownership changes as a result of future transactions in our stock, which may be outside our control, then our ability to utilize NOLs and other pre-change tax attributes could be further limited by Sections 382 and 383 of the Code, and certain of our NOLs and other pre-change tax attributes may expire unused. As a result, if or when we earn net taxable income, our ability to use our pre-change NOLs or other tax attributes to offset such taxable income or otherwise reduce any liability for income taxes may be subject to limitations, which could adversely affect our future cash flows.
30
Unfavorable global economic conditions and adverse developments with respect to financial institutions and associated liquidity risk could adversely affect our business, financial condition and stock price.
The global credit and financial markets are currently, and have from time to time experienced extreme volatility and disruptions, including severely diminished liquidity and credit availability, rising interest and inflation rates, declines in consumer confidence, declines in economic growth, increases in unemployment rates and uncertainty about economic stability. The financial markets and the global economy may also be adversely affected by the current or anticipated impact of military conflict, including the ongoing conflict between Russia and Ukraine, terrorism or other geopolitical events. Sanctions imposed by the United States and other countries in response to such conflicts, including the one in Ukraine, may also adversely impact the financial markets and the global economy, and any economic countermeasures by the affected countries or others could exacerbate market and economic instability. More recently, the closures of Silicon Valley Bank, or SVB, and Signature Bank and their placement into receivership with the Federal Deposit Insurance Corporation, or FDIC created bank-specific and broader financial institution liquidity risk and concerns. Although the Department of the Treasury, the Federal Reserve, and the FDIC jointly released a statement that depositors at SVB and Signature Bank would have access to their funds, even those in excess of the standard FDIC insurance limits, under a systemic risk exception, future adverse developments with respect to specific financial institutions or the broader financial services industry may lead to market-wide liquidity shortages, impair the ability of companies to access near-term working capital needs, and create additional market and economic uncertainty. There can be no assurance that future credit and financial market instability and a deterioration in confidence in economic conditions will not occur. Our general business strategy may be adversely affected by any such economic downturn, liquidity shortages, volatile business environment or continued unpredictable and unstable market conditions. If the equity and credit markets deteriorate, or if adverse developments are experienced by financial institutions, it may cause short-term liquidity risk and also make any necessary debt or equity financing more difficult, more costly, more onerous with respect to financial and operating covenants and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance and stock price and could require us to delay or abandon clinical development plans. In addition, there is a risk that one or more of our current service providers, financial institutions, manufacturers and other partners may be adversely affected by the foregoing risks, which could directly affect our ability to attain our operating goals on schedule and on budget.
If our labor costs continue to rise, including due to shortages, changes in certification requirements and/or higher than normal turnover rates in skilled clinical personnel; or currently pending or future governmental laws, rules, regulations or initiatives impose additional requirements or limitations on our operations or profitability; or, if we are unable to attract and retain key leadership talent, we may experience disruptions in our business operations and increases in operating expenses, among other things, which could have a material adverse effect on our business, results of operations, financial condition and cash flows.
We have incurred and expect to continue to incur increased labor costs and experience staffing challenges related to COVID-19, the extent of which will depend on the severity and duration of the pandemic, among other things. Furthermore, changes in certification requirements can impact our ability to maintain sufficient staff levels, including to the extent our teammates are not able to meet new requirements, among other things. In addition, if we experience a higher-than-normal turnover rate for our skilled clinical personnel, our operations and treatment growth may be negatively impacted, which could adversely affect our business, results of operations, financial condition and cash flows. We also face competition in attracting and retaining talent for key leadership positions. If we are unable to attract and retain qualified individuals, we may experience disruptions in our business operations, including, without limitation, our ability to achieve strategic goals, which could have a material adverse effect on our business, results of operations, financial condition and cash flows.
Adverse developments affecting financial institutions, companies in the financial services industry or the financial services industry generally, such as actual events or concerns involving liquidity, defaults or non-performance, could adversely affect our operations and liquidity.
Actual events involving limited liquidity, defaults, non-performance or other adverse developments that affect financial institutions or other companies in the financial services industry or the financial services industry generally, or concerns or rumors about any events of these kinds, have in the past and may in the future lead to market-wide liquidity problems.
Our access to our cash and cash equivalents in amounts adequate to finance our operations could be significantly impaired by the financial institutions with which we have arrangements directly facing liquidity constraints or failures. In addition, investor concerns regarding the U.S. or international financial systems could result in less favorable commercial financing terms, including higher interest rates or costs and tighter financial and operating covenants, or systemic limitations on access to credit and liquidity sources, thereby making it more difficult for us to acquire financing on acceptable terms or at all. Any material decline in available funding or our ability to access our cash and cash equivalents could adversely impact our ability to meet our operating expenses, result in breaches of our contractual obligations or result in violations of federal or state wage and hour laws, any of which could have material adverse impacts on our operations and liquidity.
31
Risks Related to Development, Clinical Testing, Manufacturing, Regulatory Approval and Commercialization
Clinical trials are expensive, time-consuming and difficult to design and implement, and involve an uncertain outcome.
Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. Because the results of preclinical studies and early clinical trials are not necessarily predictive of future results, our product candidates may not have favorable results in later preclinical and clinical studies or receive regulatory approval. We may experience delays in initiating and completing any clinical trials that we intend to conduct, and we do not know whether planned clinical trials will begin on time, need to be redesigned, enroll patients on time or be completed on schedule, or at all. Clinical trials can be delayed for a variety of reasons, including delays related to:
| ● | the FDA or comparable foreign regulatory authorities disagreeing as to the design or implementation of our clinical studies; |
| ● | obtaining regulatory approval to commence a trial; |
| ● | reaching an agreement on acceptable terms with prospective CROs, and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| ● | obtaining Institutional Review Board (“IRB”), approval at each site, or Independent Ethics Committee (“IEC”), approval at sites outside the United States; |
| ● | recruiting suitable patients to participate in a trial in a timely manner and in sufficient numbers; |
| ● | having patients complete a trial or return for post-treatment follow-up; |
| ● | imposition of a clinical hold by regulatory authorities, including as a result of unforeseen safety issues or side effects or failure of trial sites to adhere to regulatory requirements or follow trial protocols; |
| ● | clinical sites deviating from trial protocol or dropping out of a trial; |
| ● | addressing patient safety concerns that arise during the course of a trial; |
| ● | adding a sufficient number of clinical trial sites; or |
| ● | manufacturing sufficient quantities of product candidate for use in clinical trials. |
We could also encounter delays if a clinical trial is suspended or terminated by us, the IRBs or IECs of the institutions in which such trials are being conducted, the Data Safety Monitoring Board (“DSMB”) for such trial or the FDA or other regulatory authorities. Such authorities may impose such a suspension or termination due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. Furthermore, we rely on CROs and clinical trial sites to ensure the proper and timely conduct of our clinical trials and, while we have agreements governing their committed activities, we have limited influence over their actual performance, as described in “Risks Related to Our Dependence on Third Parties”.
32
The regulatory approval processes of the FDA and comparable foreign authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidates, our business will be substantially harmed.
The time required to obtain approval by the FDA and comparable foreign authorities is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, regulations or the type and amount of clinical data necessary to gain regulatory approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. We have not obtained regulatory approval for any product candidate and it is possible that we will never obtain regulatory approval for our product candidates. We are not permitted to market any of our product candidates in the United States until we receive regulatory approval of an NDA from the FDA. Our product candidates could fail to receive regulatory approval for many reasons, including the following:
| ● | we may be unable to demonstrate to the satisfaction of the FDA or comparable foreign regulatory authorities that a product candidate is safe and effective for its proposed indication; |
| ● | serious and unexpected drug-related side effects experienced by participants in our clinical trials or by individuals using drugs similar to our product candidates, or other products containing the active ingredient in our product candidates; |
| ● | negative or ambiguous results from our clinical trials or results that may not meet the level of statistical significance required by the FDA or comparable foreign regulatory authorities for approval; |
| ● | we may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
| ● | the FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials; |
| ● | the data collected from clinical trials of our product candidates may not be acceptable or sufficient to support the submission of an NDA or other submission or to obtain regulatory approval in the United States or elsewhere, and we may be required to conduct additional clinical trials; |
| ● | the FDA or comparable foreign authorities may disagree regarding the formulation, labeling and/or the specifications of our product candidates; |
| ● | the FDA or comparable foreign regulatory authorities may fail to approve or find deficiencies with the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and |
| ● | the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval. |
Prior to obtaining approval to commercialize a product candidate in the United States or abroad, we must demonstrate with substantial evidence from well-controlled clinical trials, and to the satisfaction of the FDA or foreign regulatory agencies, that such product candidates are safe and effective for their intended uses. Results from preclinical studies and clinical trials can be interpreted in different ways. Even if we believe the preclinical or clinical data for our product candidates are promising, such data may not be sufficient to support approval by the FDA and other regulatory authorities.
33
The FDA or any foreign regulatory bodies can delay, limit or deny approval of our product candidates or require us to conduct additional preclinical or clinical testing or abandon a program for many reasons, including:
| ● | the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical trials; |
| ● | the FDA or comparable foreign regulatory authorities may disagree with our safety interpretation of our product candidate; |
| ● | the FDA or comparable foreign regulatory authorities may disagree with our efficacy interpretation of our product candidate; |
| ● | the FDA or comparable foreign regulatory authorities may regard our CMC package as inadequate. |
Of the large number of drugs in development, only a small percentage successfully complete the regulatory approval processes and are commercialized. This lengthy approval process, as well as the unpredictability of future clinical trial results, may result in our failing to obtain regulatory approval to market our product candidates, which would significantly harm our business, results of operations and prospects.
In addition, the FDA or the applicable foreign regulatory agency also may approve a product candidate for a more limited indication or patient population than we originally requested, and the FDA or applicable foreign regulatory agency may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate. Any of the foregoing scenarios could materially harm the commercial prospects for our product candidates.
We may encounter substantial delays in our planned clinical trials, or may not be able to conduct or complete our clinical trials on the timelines we expect, if at all.
Our planned clinical trials are expected to be expensive, time consuming, and subject to uncertainty. We cannot guarantee that any clinical trials will be conducted as planned or completed on schedule, if at all. We cannot be sure that submission of an IND or, in the case of the European Medicines Agency (the “EMA”), a clinical trial application (a “CTA”), will result in the FDA or EMA allowing clinical trials to begin in a timely manner, if at all. Moreover, even if these trials begin, issues may arise that could suspend or terminate such clinical trials. A failure of one or more clinical trials can occur at any stage of testing, and our future clinical trials may not be successful. Events that may prevent successful or timely initiation or completion of clinical trials include:
| ● | inability to generate sufficient preclinical, toxicology, or other in vivo or in vitro data to support the initiation or continuation of clinical trials; |
| ● | delays in confirming target engagement, patient selection or other relevant biomarkers to be utilized in preclinical and clinical product candidate development; |
| ● | delays in reaching a consensus with regulatory agencies on study design; |
| ● | delays in reaching agreement on acceptable terms with prospective contract research organizations (“CROs”) and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and clinical trial sites; |
| ● | delays in identifying, recruiting and training suitable clinical investigators; |
| ● | delays in obtaining required IRB approval at each clinical trial site; |
34
| ● | imposition of a temporary or permanent clinical hold by regulatory agencies for a number of reasons, including, but not limited to, after review of an IND or amendment, CTA or amendment, or equivalent application or amendment; as a result of a new safety finding that presents unreasonable risk to clinical trial participants; a negative finding from an inspection of our clinical trial operations or study sites; developments in trials conducted by competitors that raise FDA or EMA concerns about risk to patients broadly; or if the FDA or EMA finds that the investigational protocol or plan is clearly deficient to meet its stated objectives; |
| ● | delays or difficulties resulting from the COVID-19 pandemic; |
| ● | delays in identifying, recruiting and enrolling suitable patients to participate in our clinical trials, and delays caused by patients withdrawing from clinical trials or failing to return for post-treatment follow-up; |
| ● | difficulty collaborating with patient groups and investigators; |
| ● | failure by our CROs, other third parties, or us to adhere to clinical trial requirements; |
| ● | failure to perform in accordance with the FDA’s or any other regulatory authority’s current good clinical practices, requirements, or applicable EMA or other regulatory guidelines in other countries; |
| ● | occurrence of adverse events associated with a product candidate that are viewed to outweigh its potential benefits; |
| ● | changes in regulatory requirements and guidance that require amending or submitting new clinical protocols; |
| ● | changes in the standard of care on which a clinical development plan was based, which may require new or additional trials; |
| ● | the cost of clinical trials of our product candidates being greater than we anticipate; |
| ● | clinical trials of our product candidates producing negative or inconclusive results, which may result in our deciding, or regulators requiring us, to conduct additional clinical trials or abandon product development programs; and |
| ● | delays in manufacturing, testing, releasing, validating, or importing/exporting sufficient stable quantities of our product candidates for use in clinical trials or the inability to do any of the foregoing. |
Any inability to successfully initiate or complete future clinical trials could result in additional costs to us or impair our ability to generate revenue. In addition, if we make manufacturing or formulation changes to our product candidates, we may be required to or we may elect to conduct additional studies to bridge our modified product candidates to earlier versions. Clinical trial delays could also shorten any periods during which our products have patent protection and may allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our product candidates and may harm our business and results of operations.
We could also encounter delays if a clinical trial is suspended or terminated by us, by the data safety monitoring board for such trial or by the FDA, EMA or any other regulatory authority, or if the IRBs of the institutions in which such trials are being conducted suspend or terminate the participation of their clinical investigators and sites subject to their review. Such authorities may suspend or terminate a clinical trial due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA, EMA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a product candidate, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial.
35
Our preclinical programs may experience delays or may never advance to clinical trials, which would adversely affect our ability to obtain regulatory approvals or commercialize these programs on a timely basis or at all.
In order to obtain FDA or other regulatory authority approval to market a new biological product we must demonstrate proof of safety, purity and potency, and efficacy in humans. To meet these requirements we will have to conduct adequate and well-controlled clinical trials. Before we can commence clinical trials for a product candidate, we must complete extensive preclinical testing and studies that support our planned INDs in the United States. We cannot be certain of the timely completion or outcome of our preclinical testing and studies and cannot predict if the FDA will accept our proposed clinical programs or if the outcome of our preclinical testing and studies will ultimately support the further development of our programs. As a result, we cannot be sure that we will be able to submit INDs or similar applications for our preclinical programs on the timelines we expect, if at all, and we cannot be sure that submission of INDs or similar applications will result in the FDA or other regulatory authorities allowing clinical trials to begin.
Conducting preclinical testing is a lengthy, time-consuming and expensive process. The length of time may vary substantially according to the type, complexity and novelty of the program, and often can be several years or more per program. Any delays in preclinical testing and studies conducted by us or potential future partners may cause us to incur additional operating expenses. The commencement and rate of completion of preclinical studies and clinical trials for a product candidate may be delayed by many factors, including, for example:
| ● | inability to generate sufficient preclinical or other in vivo or in vitro data to support the initiation of clinical trials; |
| ● | delays in reaching a consensus with regulatory agencies on study design; and |
| ● | the FDA not allowing us to rely on previous findings of safety and efficacy for other similar but approved products and published scientific literature. |
Moreover, because standards for pre-clinical assessment are evolving and may change rapidly, even if we reach an agreement with the FDA on a pre-IND proposal, the FDA may not accept the IND submission as presented, in which case patient enrollment would be placed on partial or complete hold and treatment of enrolled patients could be discontinued while the product candidate is re-evaluated. Even if clinical trials do begin for our preclinical programs, our clinical trials or development efforts may not be successful.
We may attempt to secure approval from the FDA or comparable foreign regulatory authorities through an expedited review program, and if we are unable to do so, then we could face increased expense to obtain, and delays in the receipt of, necessary marketing approvals.
We may in the future seek approval for one or more of our future product candidates under one of the FDA’s expedited review programs for serious conditions. These programs are available to sponsors of therapies that address an unmet medical need to treat a serious condition. The qualifying criteria and requirements vary for each expedited program. Prior to seeking review under one of these expedited programs for any of our future product candidates, we intend to seek feedback from the FDA and will otherwise evaluate our ability to seek and receive marketing approval through an expedited review program.
There can be no assurance that, after our evaluation of the FDA’s feedback and other factors, we will decide to pursue one or more of these expedited review programs. Similarly, there can be no assurance that after subsequent FDA feedback we will continue to pursue one or more of these expedited programs, even if we initially decide to do so. Furthermore, FDA could decide not to grant our request to use one or more of the expedited review programs for a product candidate, even if the FDA’s initial feedback is that the product candidate would qualify for such program(s). Moreover, FDA can decide to stop reviewing a product candidate under one or more of these expedited review programs if, for example, the conditions that warranted expedited review no longer apply to that product candidate.
Some of these expedited programs (e.g., accelerated approval) also require post-marketing clinical trials to be completed and, if any such required trial fails, the FDA could withdraw the approval of the product. If one of our future product candidates does not qualify for any expedited review program, then this could result in a longer time period to approval and commercialization of such product candidate, could increase the cost of development of such product candidate, and could harm our competitive position in the marketplace.
36
We may seek Orphan Drug Designation for our product candidates, and we may be unsuccessful or may be unable to maintain the benefits associated with Orphan Drug Designation, including the potential for market exclusivity.
We have received Orphan Drug Designation for our PAS-004 product candidate for the treatment of NF1. Regulatory authorities in some jurisdictions, including the United States and Europe, may designate drugs for relatively small patient populations as orphan drugs. Under the Orphan Drug Act, the FDA may designate a drug as an orphan drug if it is a drug intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals annually in the United States, or a patient population greater than 200,000 in the United States where there is no reasonable expectation that the cost of developing the drug will be recovered from sales in the United States. In the United States, Orphan Drug Designation may entitle a party to financial incentives such as grant funding towards clinical trial costs, tax advantages and user-fee waivers.
Similarly, in Europe, the European Commission grants Orphan Drug Designation after receiving the opinion of the EMA Committee for Orphan Medicinal Products on an Orphan Drug Designation application. Orphan Drug Designation is intended to promote the development of drugs that are intended for the diagnosis, prevention or treatment of life-threatening or chronically debilitating conditions affecting not more than 5 in 10,000 persons in Europe and for which no satisfactory method of diagnosis, prevention, or treatment has been authorized (or the product would be a significant benefit to those affected). Additionally, designation is granted for drugs intended for the diagnosis, prevention, or treatment of a life-threatening, seriously debilitating or serious and chronic condition and when, without incentives, it is unlikely that sales of the drug in Europe would be sufficient to justify the necessary investment in developing the drug. In Europe, Orphan Drug Designation may entitle a party to a number of incentives, such as protocol assistance and scientific advice specifically for designated orphan medicines, and potential fee reductions depending on the status of the sponsor.
Generally, if a drug with an Orphan Drug Designation subsequently receives the first marketing approval for the indication for which it has such designation, the drug is entitled to a period of marketing exclusivity, which precludes the EMA or the FDA from approving another marketing application for the same drug and indication for that time period, except in limited circumstances. The applicable period is seven years in the United States and ten years in Europe. The European exclusivity period can be reduced to six years if a drug no longer meets the criteria for Orphan Drug Designation or if the drug is sufficiently profitable such that market exclusivity is no longer justified.
Even if we obtain orphan drug exclusivity for our product candidates, that exclusivity may not effectively protect those product candidates from competition because different therapies can be approved for the same condition and the same therapies can be approved for different conditions but used off-label. Even after an orphan drug is approved, the FDA can subsequently approve another drug for the same condition if the FDA concludes that the later drug is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care. In addition, a designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. Moreover, orphan drug exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition. Orphan Drug Designation neither shortens the development time or regulatory review time of a drug nor gives the drug any advantage in the regulatory review or approval process. While we may seek Orphan Drug Designation for applicable indications for our product candidates, we may never receive such designations. Even if we do receive such designations, there is no guarantee that we will enjoy the benefits of those designations.
37
We may not identify or discover other product candidates and may fail to capitalize on programs or product candidates that may present a greater commercial opportunity or for which there is a greater likelihood of success.
Our business depends upon our ability to identify, develop and commercialize product candidates. A key element of our strategy is to discover and develop additional product candidates based upon our Treg Modalities. We are seeking to do so through our internal research programs, and may also explore strategic collaborations for the discovery of new product candidates. Research programs to identify product candidates require substantial technical, financial and human resources, whether or not any product candidates are ultimately identified. In addition, targets for different neurodegenerative and auto immune diseases may require changes to our cell manufacturing platform, which may slow down development or make it impossible to manufacture our product candidates. Our research programs may initially show promise in identifying potential product candidates, yet fail to yield product candidates for clinical development for many reasons, including the following:
| ● | the research methodology or technology modality used may not be successful in identifying potential product candidates; |
| ● | competitors may develop alternatives that render our product candidates obsolete or less attractive; |
| ● | we may choose to cease development if we determine that clinical results do not show promise; |
| ● | product candidates we develop may nevertheless be covered by third-party patents or other exclusive rights; |
| ● | a product candidate may be shown to have harmful side effects or other characteristics that indicate it is unlikely to be effective or otherwise does not meet applicable regulatory criteria; and |
| ● | a product candidate may not be accepted as safe and effective by patients, the medical community or third-party payors. |
Because we have limited resources, we must choose to pursue and fund the development of specific types of treatment, and we may forego or delay pursuit of opportunities with certain programs or product candidates or for indications that later prove to have greater commercial potential. Our estimates regarding the potential market for our product candidates could be inaccurate, and if we do not accurately evaluate the commercial potential for a particular product candidate, we may relinquish valuable rights to that product candidate through strategic collaboration, licensing or other arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate. Alternatively, we may allocate internal resources to a product candidate in a therapeutic area in which it would have been more advantageous to enter into a partnering arrangement.
If any of these events occur, we may be forced to abandon or delay our development efforts with respect to a particular product candidate or fail to develop a potentially successful product candidate.
If any of our product candidates are approved for marketing and commercialization and we have not developed or secured third- party marketing, sales and distribution capabilities, we will be unable to successfully commercialize such products and may not be able to generate product revenue.
We currently have no sales, marketing or distribution organizational experience or capabilities. We will need to develop internal sales, marketing and distribution capabilities to commercialize any product candidate that gains FDA or other regulatory authority approval, which would be expensive and time-consuming, or enter into partnerships with third parties to perform these services. If we decide to market any approved products directly, we will need to commit significant financial and managerial resources to develop a marketing and sales force with technical expertise and supporting distribution, administration and compliance capabilities. If we rely on third parties to market products or decide to co-promote products with partners, we will need to establish and maintain marketing and distribution arrangements with third parties, and there can be no assurance that we will be able to enter into such arrangements on acceptable terms or at all.
38
We will face significant competition in seeking appropriate strategic partners and the negotiation process is time-consuming and complex. Whether we reach a definitive agreement for other collaborations will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design or results of clinical trials, the progress of our clinical trials, the likelihood of approval by the FDA or similar regulatory authorities outside the United States, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available to collaborate on and whether such a collaboration could be more attractive than the one with us for our product candidate. Further, we may not be successful in our efforts to establish a strategic partnership or other alternative arrangements for future product candidates because they may be deemed to be at too early of a stage of development for collaborative effort and third parties may not view them as having the requisite potential to demonstrate safety and efficacy. Any delays in entering into new collaborations or strategic partnership agreements related to any product candidate we develop could delay the development and commercialization of our product candidates, which would harm our business prospects, financial condition, and results of operations.
The FDA and other regulatory agencies actively enforce the laws and regulations prohibiting pre-approval promotion and the promotion of off-label uses.
The FDA prohibits the pre-approval promotion of drugs as safe and effective for the purposes for which they are under investigation. Similarly, the FDA prohibits the promotion of approved drugs for new or unapproved indications. If the FDA finds that we have engaged in pre-approval promotion of our future product candidates, or if any of our future product candidates are approved and we are found to have improperly promoted off-label uses of those products, we may become subject to significant liability. The FDA and other regulatory agencies strictly regulate the promotional claims that may be made about prescription products, such as our future product candidates, if approved. In particular, an approved product may not be promoted for uses that are not approved by the FDA or such other regulatory agencies as reflected in the product’s approved labeling. If we receive marketing approval for a product candidate, physicians may nevertheless prescribe it to their patients in a manner that is inconsistent with the approved label, which is within their purview as part of their practice of medicine. If we are found to have promoted such off-label uses, however, we may become subject to significant liability. The U.S. federal government has levied large civil and criminal fines against companies for alleged improper promotion of off-label use and has enjoined several companies from engaging in off-label promotion. The FDA has also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed. The FDA may also issue a public warning letter or untitled letter to the company. If we cannot successfully manage the promotion of our future approved products, we could become subject to significant liability, which would materially adversely affect our business and financial condition.
Our business activities may be subject to the U.S. Foreign Corrupt Practices Act, or the FCPA, and similar anti-bribery and anti-corruption laws of other countries in which we operate, as well as U.S. and certain foreign export controls, trade sanctions, and import laws and regulations. Compliance with these legal requirements could limit our ability to compete in foreign markets and subject us to liability if we violate them.
If we further expand our operations outside of the United States, we must dedicate additional resources to comply with numerous laws and regulations in each jurisdiction in which we plan to operate. Our business activities may be subject to the FCPA and similar anti-bribery or anti-corruption laws, regulations or rules of other countries in which we operate. The FCPA generally prohibits companies and their employees and third-party intermediaries from offering, promising, giving or authorizing the provision of anything of value, either directly or indirectly, to a non-U.S. government official in order to influence official action or otherwise obtain or retain business. The FCPA also requires public companies to make and keep books and records that accurately and fairly reflect the transactions of the corporation and to devise and maintain an adequate system of internal accounting controls. Our business is heavily regulated and therefore involves significant interaction with public officials, including officials of non-U.S. governments. Additionally, in many other countries, hospitals owned and operated by the government, and doctors and other hospital employees would be considered foreign officials under the FCPA. Recently the Securities and Exchange Commission (“SEC”) and Department of Justice (“DOJ”) have increased their FCPA enforcement activities with respect to biotechnology and pharmaceutical companies. There is no certainty that all of our employees, agents or contractors, or those of our affiliates, will comply with all applicable laws and regulations, particularly given the high level of complexity of these laws. Violations of these laws and regulations could result in fines, criminal sanctions against us, our officers or our employees, disgorgement, and other sanctions and remedial measures, and prohibitions on the conduct of our business. Any such violations could include prohibitions on our ability to offer our products in one or more countries and could materially damage our reputation, our brand, our international activities, our ability to attract and retain employees and our business, prospects, operating results and financial condition.
39
In addition, our products and technology may be subject to U.S. and foreign export controls, trade sanctions and import laws and regulations. Governmental regulation of the import or export of our products and technology, or our failure to obtain any required import or export authorization for our products, when applicable, could harm our international sales and adversely affect our revenue. Compliance with applicable regulatory requirements regarding the export of our products may create delays in the introduction of our products in international markets or, in some cases, prevent the export of our products to some countries altogether. Furthermore, U.S. export control laws and economic sanctions prohibit the shipment of certain products and services to countries, governments, and persons targeted by U.S. sanctions. If we fail to comply with export and import regulations and such economic sanctions, penalties could be imposed, including fines and/or denial of certain export privileges. Moreover, any new export or import restrictions, new legislation or shifting approaches in the enforcement or scope of existing regulations, or in the countries, persons, or products targeted by such regulations, could result in decreased use of our products by, or in our decreased ability to export our products to existing or potential customers with international operations. Any decreased use of our products or limitation on our ability to export or sell access to our products would likely adversely affect our business.
Our business involves the use of hazardous materials and we and our third-party manufacturers and suppliers must comply with environmental laws and regulations, which can be expensive and restrict how we do business.
Our research and development activities and our third-party manufacturers’ and suppliers’ activities involve the controlled storage, use and disposal of hazardous materials owned by us. We and our manufacturers and suppliers are subject to laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. In some cases, these hazardous materials and various wastes resulting from their use are stored at our manufacturers’ facilities pending their use and disposal.
We cannot eliminate the risk of contamination, which could cause an interruption of our research and development efforts and business operations, environmental damage resulting in costly clean-up and liabilities under applicable laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. Although we believe that the safety procedures utilized by our third-party manufacturers and suppliers for handling and disposing of these materials generally comply with the standards prescribed by these laws and regulations, we cannot guarantee that this is the case or eliminate the risk of accidental contamination or injury from these materials. In such an event, we may be held liable for any resulting damages and such liability could exceed our resources and state or federal or other applicable authorities may curtail our use of certain materials and/or interrupt our business operations. Furthermore, environmental laws and regulations are complex, change frequently and have tended to become more stringent over time. We cannot predict the impact of such changes and cannot be certain of our future compliance. We do not currently carry biological or hazardous waste insurance coverage. Any contamination by such hazardous materials could therefore materially adversely affect our business, financial condition, results of operations and growth prospects.
Disruptions in the global economy and supply chains may have a material adverse effect on our business, financial condition and results of operations.
The disruptions to the global economy which began in 2020 have impeded global supply chains, resulting in longer lead times and also increased critical component costs and freight expenses. We have taken and may have to take steps to minimize the impact of these disruptions in lead times and increased costs by working closely with our suppliers and other third parties on whom we rely for the conduct of our business. Despite the actions we may have to undertake to minimize the impacts from disruptions to the global economy, there can be no assurances that unforeseen future events in the global supply chain will not have a material adverse effect on our business, financial condition and results of operations.
Furthermore, inflation can adversely affect us by increasing the costs of clinical trials, the research and development of our product candidates, as well as administration and other costs of doing business. We may experience increases in the prices of labor and other costs of doing business. In an inflationary environment, cost increases may outpace our expectations, causing us to use our cash and other liquid assets faster than forecasted. If this happens, we may need to raise additional capital to fund our operations, which may not be available in sufficient amounts or on reasonable terms, if at all, sooner than expected.
40
Risks Related to Our Dependence on Third Parties
We rely completely on third parties to supply drug substance and manufacture drug product for our clinical trials and preclinical studies. We intend to rely on other third parties to produce commercial supplies of product candidates, and our dependence on third parties could adversely impact our business.
We are completely dependent on third-party suppliers of the drug substance and drug product for our product candidates. If third-party suppliers do not supply sufficient quantities of materials to us on a timely basis and in accordance with applicable specifications and other regulatory requirements, there could be a significant interruption of our supplies, which would adversely affect clinical development and commercialization. Furthermore, if any of our contract manufacturers cannot successfully manufacture material that conforms to our specifications within regulatory requirements, we will not be able to secure and/or maintain regulatory approval, if any, for our product candidates.
We currently only use one CMO for the production of PAS-004 drug substance and we plan to utilize the same manufacturer for the production of drug product for our clinical trials. The termination of this relationship would result in a disruption to our product development and our business may be harmed.
We also rely on our contract manufacturers to purchase from third-party suppliers the materials necessary to produce our product candidates for our anticipated clinical trials. We do not have any control over the process or timing of the acquisition of raw materials by our contract manufacturers. Moreover, we currently do not have agreements in place for the commercial production of these raw materials. Any significant delay in the supply of a product candidate or the raw material components thereof for an ongoing clinical trial, including as a result of the COVID-19 pandemic or the conflict between Russia and Ukraine, could considerably delay completion of that clinical trial, product candidate testing, and potential regulatory approval of that product candidate.
We do not expect to have the resources or capacity to commercially manufacture any of our proposed product candidates if approved and will likely continue to be dependent on third-party manufacturers. Our dependence on third parties to manufacture and supply clinical trial materials and any approved product candidates may adversely affect our ability to develop and commercialize our product candidates on a timely basis.
We have in the past relied and expect to continue to rely on third-party CROs and other third parties to conduct and oversee our research programs, preclinical studies, planned clinical trials and other aspects of product development. If these third parties do not meet our requirements or otherwise operate as required, we may not be able to satisfy our contractual obligations or obtain regulatory approval for, or commercialize, our product candidates when expected or at all.
We have in the past relied and expect to continue to rely on third-party CROs to conduct and oversee our research programs, preclinical studies, clinical trials and other aspects of product development. We will also rely upon various medical institutions, clinical investigators and contract laboratories to conduct our trials in accordance with our clinical protocols and all applicable regulatory requirements, including the FDA’s regulations and GCPs, which are an international standard meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, administrators and monitors, and state regulations governing the handling, storage, security and recordkeeping for drug and biologic products. These CROs and other third parties will play a significant role in the conduct of these trials and the subsequent collection and analysis of data from our planned clinical trials. We will rely heavily on these parties for the execution of our clinical trials and preclinical studies, and control only certain aspects of their activities. We and our CROs and other third-party contractors are required to comply with GCP, GLP, and GACP requirements, which are regulations and guidelines enforced by the FDA and comparable foreign regulatory authorities for products in clinical development. Regulatory authorities enforce these GCP, GLP and GACP requirements through periodic inspections of trial sponsors, principal investigators and trial sites. If we or any of these third parties fail to comply with applicable GCP, GLP and GACP requirements, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or other regulatory authority may require us to perform additional clinical trials before approving our or our partners’ marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical or preclinical trials complies with applicable GCP and GLP requirements. In addition, our clinical trials must generally be conducted with product produced under cGMP regulations. Our failure to comply with these regulations and policies may require us to repeat clinical trials, which would delay the regulatory approval process.
41
Our CROs are not our employees, and we do not control whether or not they devote sufficient time and resources to our preclinical or clinical trials. Our CROs may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials, or other drug development activities, which could harm our competitive position. We face the risk of potential unauthorized disclosure or misappropriation of our intellectual property by CROs, which may reduce our trade secret protection and allow our potential competitors to access and exploit our proprietary technology. If our CROs do not successfully carry out their contractual duties or obligations, fail to meet expected deadlines, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for any other reason, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for, or successfully commercialize any product candidate that we develop. As a result, our financial results and the commercial prospects for any product candidate that we may develop would be harmed, our costs could increase, and our ability to generate revenue could be delayed.
If any of our CROs or clinical trial sites terminate their involvement in one of our preclinical studies or clinical trials for any reason, we may not be able to enter into arrangements with alternative CROs or clinical trial sites, or do so on commercially reasonable terms. In addition, if our relationship with clinical trial sites is terminated, we may experience the loss of follow-up information on patients unless we are able to transfer the care of those patients to another qualified clinical trial site. In addition, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and could receive cash or equity compensation in connection with such services. If these relationships and any related compensation result in perceived or actual conflicts of interest, the integrity of the data generated at the applicable clinical trial site may be questioned by the FDA.
We also rely on research institutions to conduct our research programs, preclinical studies and planned clinical trials. Our reliance upon research institutions, including hospitals and clinics, provides us with less control over the timing and cost of clinical trials and the ability to recruit subjects. If we are unable to reach agreement with suitable research institutions on acceptable terms, or if any resulting agreement is terminated, we may be unable to quickly replace the research institution with another qualified institution on acceptable terms. Even if we do replace the institution, we may incur additional costs to conduct the trial at the new institution. We may not be able to secure and maintain suitable research institutions to conduct our clinical trials.
If we enter into collaborations with third parties to develop or commercialize our product candidates, our prospects with respect to those product candidates will depend in significant part on the success of those collaborations.
If we enter into future collaboration with third parties, we could face the following risks:
| ● | collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations; |
| ● | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates; |
| ● | collaborators may not properly enforce, maintain or defend our intellectual property rights or may use our proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation, or other intellectual property proceedings; |
| ● | disputes may arise between a collaborator and us that cause the delay or termination of the research, development or commercialization of the product candidate, or that result in costly litigation or arbitration that diverts management attention and resources; |
| ● | if a present or future collaborator of ours were to be involved in a business combination, the continued pursuit and emphasis on our product development or commercialization program under such collaboration could be delayed, diminished or terminated; and |
| ● | collaboration agreements may restrict our right to independently pursue new product candidates. |
42
If conflicts arise between our collaborators and us, our collaborators may act in a manner adverse to us and could limit our ability to implement our strategies. Future collaborators may develop, either alone or with others, products in related fields that are competitive with the products or potential products that are the subject of these collaborations. Competing products, either developed by the collaborators or to which the collaborators have rights, may result in the withdrawal of support for our product candidates. Our collaborators may preclude us from entering into collaborations with their competitors, fail to obtain timely regulatory approvals, terminate their agreements with us prematurely or fail to devote sufficient resources to the development and commercialization of products. Any of these developments could harm our product development efforts.
As a result, if we enter into additional collaboration agreements and strategic partnerships or license our intellectual property, products or businesses, we may not be able to realize the benefit of such transactions if we are unable to successfully integrate them with our existing operations, which could delay our timelines or otherwise adversely affect our business. We also cannot be certain that, following a strategic transaction or license, we will achieve the revenue or specific net income that justifies such transaction.
Risks Related to Our Securities
The price of our Common Stock and Warrants may be volatile, and you could lose all or part of your investment.
The market price of our Common Stock and Warrants are highly volatile and for the year ended December 31, 2022, the market price of our Common Stock ranged from $0.54 to $1.83 per share and the market price of our Warrants ranged from $0.02 to $0.42. The recent fluctuations in our trading price and future trading in our Common Stock and Warrants may be subject to wide fluctuations in response to a variety of factors, including the following:
| ● | the timing and results of preclinical studies and clinical trials of our future product candidates or those of our competitors; | |
| ● | the success of competitive products or announcements by potential competitors of their product development efforts; | |
| ● | regulatory actions with respect to our or our competitors’ product candidates or products; | |
| ● | actual or anticipated changes in our growth rate relative to our competitors; | |
| ● | regulatory or legal developments in the United States and other countries; | |
| ● | developments or disputes concerning patent applications, issued patents or other proprietary rights; | |
| ● | the recruitment or departure of key personnel; | |
| ● | announcements by us or our competitors of significant acquisitions, strategic collaborations, joint ventures, or capital commitments; | |
| ● | actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; | |
| ● | fluctuations in the valuation of companies perceived by investors to be comparable to us; | |
| ● | market conditions in the pharmaceutical and biotechnology sector; | |
| ● | changes in the structure of healthcare payment systems; | |
| ● | price and volume fluctuations attributable to inconsistent trading volume levels of our securities; | |
| ● | announcement or expectation of additional financing efforts; | |
| ● | sales of our Common Stock and Warrants by us, our insiders or our other stockholders; | |
| ● | expiration of market stand-off or lock-up agreements; and | |
| ● | general economic, industry and market conditions. |
These and other market and industry factors may cause the market price and demand for our Common Stock and Warrants to fluctuate substantially, regardless of our actual operating performance, which may limit or prevent investors from readily selling their shares of Common Stock or Warrants and may otherwise negatively affect the liquidity of our common stock and Warrants. In addition, the stock market in general, and Nasdaq Capital Markets and emerging growth companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. In the past, when the market price of a security has been volatile, holders of that security have instituted securities class action litigation against the company that issued the security. If any of our stockholders brought a lawsuit against us, we could incur substantial costs defending the lawsuit. Such a lawsuit could also divert the time and attention of our management.
Our Warrants may not have any value.
There can be no assurance that the market price of our Common Stock will ever equal or exceed the exercise price of our outstanding Warrants. In the event that our Common Stock price does not exceed the exercise price of the Warrants during the period when the Warrants are exercisable, the Warrants may not have any value.
43
A Warrant does not entitle the holder to any rights as common stockholders until the holder exercises the Warrant for a share of our Common Stock.
Until you acquire shares of our Common Stock upon exercise of your Warrants, your Warrants will not provide you any rights as a common stockholder. Upon exercise of your Warrants, you will be entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs after the exercise date.
If securities or industry analysts do not publish research or reports, or if they publish adverse or misleading research or reports, regarding us, our business or our market, the price and trading volume of our Common Stock and Warrants could decline.
The trading market for our Common Stock and Warrants is influenced by the research and reports that securities or industry analysts publish about us, our business or our market. We do not currently have and may never obtain research coverage by securities or industry analysts. If no or few securities or industry analysts commence coverage of us, the stock price would be negatively impacted. In the event we obtain securities or industry analyst coverage, if any of the analysts who cover us issue adverse or misleading research or reports regarding us, our business model, our future intellectual property, our stock performance or our market, or if our operating results fail to meet the expectations of analysts, the price of our Common Stock and Warrants would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause the price of our Common Stock and Warrants or trading volume to decline.
Our quarterly operating results may fluctuate significantly or may fall below the expectations of investors or securities analysts, each of which may cause our stock price to fluctuate or decline.
Our operating results are subject to quarterly fluctuations. Our net loss and other operating results are affected by numerous factors, including:
| ● | variations in the level of expense related to the ongoing development of our future product candidates or future development programs; | |
| ● | results of clinical trials, or the addition or termination of clinical trials or funding support by us or potential future partners; | |
| ● | our execution of any collaboration, licensing or similar arrangements, and the timing of payments we may make or receive under potential future arrangements or the termination or modification of any such potential future arrangements; | |
| ● | any intellectual property infringement, misappropriation or violation lawsuit or opposition, interference or cancellation proceeding in which we may become involved; | |
| ● | additions and departures of key personnel; | |
| ● | strategic decisions by us or our competitors, such as acquisitions, divestitures, spin-offs, joint ventures, strategic investments or changes in business strategy; | |
| ● | if any of our future product candidates receive regulatory approval, the terms of such approval and market acceptance and demand for such approved products; | |
| ● | regulatory developments affecting our future product candidates, or those of our competitors; and | |
| ● | changes in general market and economic conditions. |
If our quarterly operating results fall below the expectations of investors or securities analysts, the price of our Common Stock and Warrants could decline substantially. Furthermore, any quarterly fluctuations in our operating results may, in turn, cause the price of our Common Stock and Warrants to fluctuate substantially. We believe that quarterly comparisons of our financial results are not necessarily meaningful and should not be relied upon as an indication of our future performance.
44
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, stockholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our Common Stock and Warrants.
Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any testing by us conducted in connection with Section 404 of the Sarbanes-Oxley Act, or any subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our financial statements or identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our securities.
We are required to disclose changes made in our internal controls and procedures on a quarterly basis and our management is required to assess the effectiveness of these controls annually. However, for as long as we are an emerging growth company, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal controls over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act. We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.235 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of our offering; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the SEC. An independent assessment of the effectiveness of our internal controls over financial reporting could detect problems that our management’s assessment might not. Undetected material weaknesses in our internal controls over financial reporting could lead to restatements of our financial statements and require us to incur the expense of remediation.
We are an “emerging growth company,” and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our Common Stock and Warrants less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we intend to take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including:
| ● | being permitted to provide only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure in this 10-K; | |
| ● | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act; | |
| ● | not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements; | |
| ● | reduced disclosure obligations regarding executive compensation in this 10-K and our periodic reports and proxy statements; and | |
| ● | exemptions from the requirements of holding nonbinding advisory stockholder votes on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
45
We cannot predict if investors will find our securities less attractive because we may rely on these exemptions. If some investors find our securities less attractive as a result, there may be a less active trading market for our securities and the trading prices of our securities may be more volatile.
We will remain an emerging growth company until the earliest to occur of: (1) the last day of the fiscal year in which we have more than $1.235 billion in annual revenue; (2) the date we qualify as a “large accelerated filer,” with at least $700 million of equity securities held by non-affiliates; (3) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period; and (4) the last day of the fiscal year ending after the fifth anniversary of our offering.
Pursuant to the JOBS Act, as an emerging growth company, we have elected to use the extended transition period for complying with any new or revised financial accounting standards to delay adopting new or revised accounting standards until such time as those standards apply to private companies.
The requirements of being a public company may strain our resources, result in more litigation and divert management’s attention.
As a public company, we are subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, or the Dodd-Frank Act, the listing requirements of Nasdaq and other applicable securities rules and regulations. Complying with these rules and regulations increases legal and financial compliance costs, makes some activities more difficult, time consuming or costly and increases demand on our systems and resources, including management. The Exchange Act requires, among other things, that we file annual, quarterly and current reports with respect to our business and operating results. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. We are required to disclose changes made in our internal control and procedures on a quarterly basis. In order to maintain and, if required, improve our disclosure controls and procedures and internal control over financial reporting to meet this standard, significant resources and management oversight may be required. As a result, management’s attention may be diverted from other business concerns, which could adversely affect our business and operating results. We may also need to hire additional employees or engage outside consultants to comply with these requirements, which will increase our costs and expenses.
In addition, changing laws, regulations and standards relating to corporate governance and public disclosure are creating uncertainty for public companies, increasing legal and financial compliance costs and making some activities more time consuming. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management’s time and attention from revenue-generating activities to compliance activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to their application and practice, regulatory authorities may initiate legal proceedings against us and our business may be adversely affected.
These new rules and regulations may make it more expensive for us to obtain director and officer liability insurance and, in the future, we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified members of our Board, particularly to serve on our Audit Committee and compensation committee (“Compensation Committee”), and qualified executive officers.
By disclosing information in this 10-K and in future filings required of a public company, our business and financial condition will become more visible, which we believe may result in threatened or actual litigation, including by competitors and other third parties. If those claims are successful, our business could be seriously harmed. Even if the claims do not result in litigation or are resolved in our favor, the time and resources needed to resolve them could divert our management’s resources and seriously harm our business.
46
We may be subject to securities litigation, which is expensive and could divert management attention.
The market price of our Common Stock and Warrants may be volatile and, in the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business.
We do not currently intend to pay dividends on our Common Stock and, consequently, your ability to achieve a return on your investment will depend on appreciation of the value of our Common Stock.
We have never declared or paid any cash dividends on our equity securities. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Any return to stockholders will therefore be limited to any appreciation in the value of our Common Stock, which is not certain.
Provisions in our Certificate of Incorporation and Bylaws and Delaware law might discourage, delay or prevent a change in control of our company or changes in our management and, therefore, depress the market price of our securities.
Our amended and restated certificate of incorporation (“Certificate of Incorporation”), and our amended and restated bylaws (“Bylaws”) contain provisions that could depress the market price of our securities by acting to discourage, delay or prevent a change in control of our Company or changes in our management that the stockholders of our Company may deem advantageous. These provisions, among other things:
| ● | prohibit cumulative voting; | |
| ● | authorize our Board to amend the Bylaws; and | |
| ● | establish advance notice requirements for nominations for election to our Board or for proposing matters that can be acted upon by stockholders at annual stockholder meetings. |
In addition, Section 203 of the General Corporation Law of the State of Delaware, or the DGCL, prohibits a publicly-held Delaware corporation from engaging in a business combination with an interested stockholder, generally a person which together with its affiliates owns, or within the last three years has owned, 15% of our voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner.
Any provision of our Certificate of Incorporation, Bylaws or Delaware law that has the effect of delaying or preventing a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our capital stock and could also affect the price that some investors are willing to pay for our securities.
Certain beneficial owners might have control over us which could delay or prevent a change in corporate control or result in the entrenchment of management and/or the Board.
As of March 27, 2023, our officers, directors and principal stockholders, beneficially own, in the aggregate, approximately 22.6% of our outstanding Common Stock. Accordingly, these stockholders, if acting together, may have the ability to impact the outcome of matters submitted to our stockholders for approval, including the election and removal of directors and any merger, consolidation, or sale of all or substantially all of our assets. In addition, these persons may have the ability to influence the management and affairs of our Company. Accordingly, this concentration of ownership may harm the market price of our securities by:
| ● | delaying, deferring, or preventing a change in control; | |
| ● | entrenching our management and/or the Board; | |
| ● | impeding a merger, consolidation, takeover, or other business combination involving us; or | |
| ● | discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us. |
47
Exchange rate fluctuations may materially affect our results of operations and financial conditions.
In light of the international scope of our operations, fluctuations in exchange rates, particularly between the U.S. dollar, the British pound and the Euro, may adversely affect us. Although we are based in the United States, we have operations in the United Kingdom. As a result, our business may be affected by fluctuations in foreign exchange rates, which may have a significant impact on our results of operations and cash flows from period to period and the price of our Common Stock and Warrants. Currently, we do not have any exchange rate hedging arrangements in place.
Failure to comply with The Nasdaq Global Market continued listing requirements may result in our Common Stock and/or Warrants being delisted from The Nasdaq Global Market.
On January 19, 2023, we received a letter from the Listing Qualifications Staff of the Nasdaq Stock Market, LLC (“Nasdaq”) indicating that, based upon the closing bid price of our Common Stock for the last 30 consecutive business days, we are not in compliance with the requirement to maintain a minimum bid price of $1.00 per share for continued listing on the Nasdaq Capital Market, as set forth in Nasdaq Listing Rule 5550(a)(2) (the “Notice”). We were provided a compliance period of 180 calendar days from the date of the Notice, or until July 18, 2023, to regain compliance with the minimum closing bid requirement, pursuant to Nasdaq Listing Rule 5810(c)(3)(A).
We will continue to monitor the closing bid price of our Common Stock and seek to regain compliance with all applicable Nasdaq requirements within the allotted compliance periods and may, if appropriate, consider available options, including implementation of a reverse stock split of our Common Stock, to regain compliance with the minimum closing bid requirement. If we seek to implement a reverse stock split in order to remain listed on Nasdaq, the announcement or implementation of such a reverse stock split could negatively affect the price of our Common Stock and/or Warrants. If we do not regain compliance within the allotted compliance periods, including any extensions that may be granted by Nasdaq, Nasdaq will provide notice that our Common Stock and Warrants will be subject to delisting. We would then be entitled to appeal that determination to a Nasdaq hearings panel. There can be no assurance that we will regain compliance with the minimum bid price requirement during the 180-day compliance period or maintain compliance with the other Nasdaq listing requirements. A delisting could substantially decrease trading in our Common Stock and Warrants, adversely affect the market liquidity of our Common Stock and Warrants as a result of the loss of market efficiencies associated with Nasdaq and the loss of federal preemption of state securities laws, adversely affect our ability to obtain financing on acceptable terms, if at all, and may result in the potential loss of confidence by investors, suppliers, customers and employees and fewer business development opportunities. Additionally, the market price of our Common Stock and/or our Warrants may decline further and stockholders may lose some or all of their investment.
Risks Related to Our Clinics Segment
Past clinical services in the US included prescribing, dispensing and administering ketamine, which as a Schedule III controlled substance under US law requires proper authorization and federal and state registration. If the clinical providers to whom we furnished business support services failed to comply with any of these requirements, we could be subject to liability and harm to our brand that would affect our business.
Ketamine is a Schedule III controlled substance under the Controlled Substances Act (“CSA”). Under the CSA, controlled substances in Schedule III have an accepted medical use in the United States and have a lower dependence and abuse potential than Schedule II substances. In order to prescribe, dispense and administer a controlled substance in Schedule III, a provider must be authorized to prescribe controlled substances by the state in which the provider is licensed and have a DEA registration.
Ketamine has been approved by the FDA for anesthetic purposes generally and, in 2019, esketamine nasal spray was approved by the FDA for treatment of treatment-resistant depression used in conjunction with an oral antidepressant. Once the FDA approves a drug, healthcare providers generally may prescribe the drug for an unapproved use when they judge that it is medically appropriate for their patient and within scope of their authority to practice. Therefore, as long as properly licensed providers are authorized to prescribe ketamine under state licensing laws, they may prescribe ketamine for “off label” uses, including for psychotherapy purposes, when deemed medically appropriate by the provider.
To be eligible for a DEA registration, practitioners must be licensed or otherwise authorized by the state in which they practice to carry out the specific activity for which they seek a DEA registration. Importantly, a physician who is registered with DEA to dispense controlled substances at a particular location in a state may travel to other unregistered locations, such as a patient’s home, in the same state to dispense controlled substances on an “as-needed and random basis,” so long as the physician does not maintain a principal place of professional practice at any of those unregistered locations. In certain states, authorized providers must also have a state specific controlled substances registration. DEA registrants may also be required to keep and submit certain records of inventory.
48
Moreover, ketamine has been identified by the DEA as a drug that has been used illegally by predators of sexual assault because it causes individuals to feel detached from their bodies and surroundings. Therefore, if our past providers who prescribed, dispensed and administered ketamine were not properly authorized and registered to do so, we could face substantial civil penalties, suffer significant reputational damage, and expose our business to other liability.
Past clinical services in the U.K. included prescribing, dispensing and administering ketamine, which as a Schedule II controlled substance under English laws requires specific manufacture, storing, and administration compliance, for an unlicensed therapeutic indication that poses certain clinical risks to patients. If certain of our past clinics and providers failed to comply with any of these requirements, we could be subject to liability and harm to our brand that may have a material adverse effect on our business.
Ketamine is a Schedule II controlled substance under the Misuse of Drugs Regulations 2001 and is controlled with regard to synthesis, storage and distribution as a Class B substance under the Misuse of Drugs Act 1971, as amended. Therefore, the associated risk factors relating to our past ownership and operation of outpatient clinics dispensing and prescribing intravenous infusions of ketamine in the U.K. include product defects that may cause liabilities under civil law for negligence and products liability under the Consumer Protection Act 1987; the medical staff operating the clinics may not have complied with standards of performance demanded by the Care Quality Commission (“CQC”) and the General Medical Council (“GMC”) code of practice; similarly the operation of the clinics themselves may not have complied with CQC rules on hygiene and safety; we may be found to not have complied with the Human Medicines Regulations 2012 with respect to advertising requirements (including the prohibition of any advertisement that is likely to lead to the use of a prescription only medicine) or the Advertising Standards Authority standards and rules (The MHRA Blue Guide on Advertising and Promotion of Medicines in the U.K. Third Edition 2020) with regard to the promotion and marketing of medicinal products; and the prescription of ketamine for the unlicensed indication of acute depressive illness may have increased the prevalence of serious adverse events, damaging the commercial reputation of our brand and future products. Additionally, we and/or associated persons may be found to not have been compliant with the Bribery Act 2010, which includes criminal liability.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
ITEM 2. PROPERTIES
We do not own any real property.
Our principal executive office is located at 1111 Lincoln Road, Suite 500, Miami Beach, FL 33139. We rent approximately 300 square feet of space, which includes our executive offices. Our research and development facility, utilized by our Therapeutics segment, is located at 458 Carlton Court, South San Francisco, CA. We rent approximately 1,900 square feet of space, which includes our laboratory and offices.
We believe that our facilities are generally in good condition and suitable to carry on our business. We also believe that, if required, suitable alternative or additional space will be available to us on commercially reasonable terms.
ITEM 3. LEGAL PROCEEDINGS
On October 31, 2022, a civil action was commenced against the Company and our Board in the Court of Chancery for the State of Delaware captioned Concord IP2 Ltd., et al. v. Pasithea Therapeutics Corp., et al., C.A. No. 2022-0980-NAC (the “Camac Action”). The Camac Action sought, among other things, a judgment declaring that the director defendants breached their fiduciary duties in connection with two acquisitions made by the Company in 2022, as well as temporary, preliminary and permanent injunctive relief enjoining the Company from counting the shares issued in connection with those two acquisitions at a special meeting of the Company’s stockholders and at the Company’s next annual meeting with respect to the election of directors.
On December 9, 2022, we entered into a Settlement and Cooperation Agreement (“Settlement Agreement”) with certain plaintiffs related to Camac Capital, LLC (the “Camac Group”). The Settlement Agreement provided, among other things, that the Camac Group would sell to the Company 3,205,282 shares of the Company’s Common Stock beneficially owned by the Camac Group at a purchase price determined by the trailing 5-day Volume-Weighted Average Price (VWAP) for the period encompassing November 30, 2022, through December 6, 2022 (which price is $1.0003 per share of Common Stock) (the “Share Repurchase”). We also agreed to reimburse the Camac Group’s expenses up to $689,491. From the date of the Cooperation agreement until the date that is three years after our 2023 annual meeting of stockholders (the “Standstill Period”), the Camac Group is subject to standstill restrictions (as more fully described in the Cooperation Agreement), including (i) support of proxy contests and other activism campaigns, calling of special meetings, and related matters (ii) participating or supporting any change of control transaction of the Company and (iii) acquiring any securities of the Company.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
49
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market information
Our Common Stock and Warrants trades on the Nasdaq Capital Market under the symbols “KTTA” and “KTTAW” respectively since September 15, 2021. Prior to that date, there was no public market for our common stock or Warrants.
Holders of Record
As of March 27, 2023, we had 44 holders of record of our Common Stock. The actual number of holders of our Common Stock is greater than this number of record holders and includes stockholders who are beneficial owners, but whose shares are held in street name by brokers or held by other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
Dividend Policy
We have never declared or paid any dividends on our Common Stock. We currently intend to retain all available funds and any future earnings, if any, to fund the development and expansion of our business, and we do not anticipate paying any cash dividends in the foreseeable future. Any future determination to pay dividends will be made at the discretion of our Board.
Repurchases
Pursuant to the Cooperation Agreement entered into in connection with the Camac Action, we repurchased from the Camac Group 3,205,282 shares of our Common Stock beneficially owned by the Camac Group, at a purchase price of $1.0003 per share. Other than the Share Repurchase, there were no repurchases of our Common Stock during the quarter ended December 31, 2022.
Use of Proceeds from Registered Securities
On September 14, 2021, our registration statement on Form S-1 (Registration No. 333-255205) was declared effective by the SEC for our initial public offering (the “Initial Public Offering”) pursuant to which we sold an aggregate of 4,800,000 units consisting of one share of our Common Stock and one Warrant to purchase one share of our Common Stock at a price to the public of $5.00 per unit, for an aggregate offering of approximately $24.0 million. EF Hutton acted as the sole book-running manager for the offering. On September 17, 2021, we closed the sale of the units, resulting in net proceeds to us of approximately $20.6 million after deducting underwriting discounts and commissions and other offering expenses. No payments were made by us to directors, officers or persons owning ten percent or more of our Common Stock or to their associates, or to our affiliates. There has been no material change in the planned use of proceeds from our Initial Public Offering as described in our final prospectus filed with the SEC on September 16, 2021 pursuant to Rule 424(b), except that we no longer plan to use any proceeds from the Initial Public Offering to expand our Clinics segment. Any proceeds that were originally intended to be used to expand the Clinics segment, and that have not already been allocated to such segment, will instead be used to wind down the Clinics segment. After the winding down of the Clinics segment, any proceeds that remain will instead be used to further develop our product candidate pipeline as part of our Therapeutics segment.
ITEM 6. [Reserved]
[Reserved]
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations is intended to provide information necessary to understand our audited consolidated financial statements for the fiscal years ended December 31, 2022 and December 31, 2021 and highlight certain other information which, in the opinion of management, will enhance a reader’s understanding of our financial condition, changes in financial condition and results of operations. In particular, the discussion is intended to provide an analysis of significant trends and material changes in our financial position and the operating results of our business during the year ended December 31, 2022, as compared to the fiscal year ended December 31, 2021. This discussion should be read in conjunction with our consolidated financial statements for the fiscal years ended December 31, 2022 and December 31, 2021 and related notes included elsewhere in this 10-K. These historical financial statements may not be indicative of our future performance. This Management’s Discussion and Analysis of Financial Condition and Results of Operations contains numerous forward-looking statements, all of which are based on our current expectations and could be affected by the uncertainties and risks described throughout this filing, particularly in “Item 1A. Risk Factors.”
50
Throughout this report, the terms “our,” “we,” “us,” and the “Company” refer to Pasithea Therapeutics Corp. and its subsidiaries, Pasithea Therapeutics Limited (UK), Pasithea Therapeutics Portugal, Sociedade Unipessoal Lda, Pasithea Clinics Inc., Alpha-5 Integrin, LLC, and AlloMek Therapeutics, LLC. Pasithea Therapeutics Limited (UK) is a private limited Company, registered in the United Kingdom (UK). Pasithea Clinics Inc. is incorporated in Delaware, Pasithea Therapeutics Portugal, Sociedade Unipessoal Lda, a private limited Company, registered in Portugal, and Alpha-5 Integrin, LLC and AlloMek Therapeutics, LLC, are both Delaware limited liability companies.
Overview
We are a biotechnology company primarily focused on the discovery, research and development of innovative treatments for central nervous system (CNS) disorders and RASopathies. Our primary operations (the “Therapeutics” segment) are focused on developing our lead therapeutic candidate, PAS-004 (CIP-137401), a macrocyclic MEK inhibitor for potential use in a range of CNS-related indications, including neurofibromatosis type 1 and Noonan syndrome as well as lamin A/C cardiomyopathy and certain oncology indications that we acquired from AlloMek Therapeutics, LLC (“AlloMek”) in October 2022. PAS-004 has displayed efficacy in a range of mouse models of various diseases and has completed pre-clinical testing and animal toxicology studies to support an Investigational New Drug application (an “IND”) with the U.S. Food and Drug Administration (“FDA”) that we plan to file in the second half of 2023 following completion of cGMP manufacturing and finalization of our toxicology program. We are also focused on the development of our discovery programs through lead identification of drug candidates, including PAS-003, a monoclonal antibody targeting a5b1 integrin for the treatment of ALS, PAS-002, a DNA vaccine targeting GlialCAM for the treatment of Multiple Sclerosis, and PAS-001, a small molecule targeting the compliment component 4 (C4) gene for the treatment of schizophrenia.
Our ability to generate product revenue will depend on the successful development, regulatory approval and eventual commercialization of one or more of our product candidates. Until such time as we can generate significant revenue from product sales, if ever, we expect to finance our operations through the sale of equity, debt financings, or other capital sources, including potential collaborations with other companies or other strategic transactions. Adequate funding may not be available to us on acceptable terms, or at all. If we fail to raise capital or enter into such agreements as and when needed, we may have to significantly delay, scale back or discontinue the development and commercialization of our product candidates.
Segments
Our business is separated into two segments, “Therapeutics” and “Clinics.”
Our Therapeutics segment performs activities related to discovery, research and development of innovative treatments for CNS disorders and other diseases. We are in the process of discontinuing our Clinics segment, which provided business support services to anti-depression clinics in the U.K. and in the United States.
The Company evaluates the performance of its business segments primarily based on revenues and net income. For the years ended December 31, 2022 and 2021, segment operating results were as follows:
| For the years ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Revenues | ||||||||
| Therapeutics | $ | - | $ | - | ||||
| Clinics | 486,559 | 15,062 | ||||||
| Total revenues | 486,559 | 15,062 | ||||||
| Net loss | ||||||||
| Therapeutics | (11,727,885 | ) | (1,908,925 | ) | ||||
| Clinics | (2,208,567 | ) | (264,596 | ) | ||||
| Total net loss | $ | (13,936,452 | ) | $ | (2,173,521 | ) | ||
51
Prior to the date of this Annual Report on Form 10-K, we have discontinued our at-home services in New York, NY as well as our services in the U.K. In addition, we have discontinued our clinical operations in Los Angeles, CA and are actively exploring options for the disposal of related property. Accordingly, as of the date of this Annual Report on Form 10-K, we have discontinued the operations of our Clinics segment.
Impact of Inflation
We have recently experienced higher costs across our business as a result of inflation, including higher costs related to employee compensation and outside services. We expect inflation to continue to have a negative impact throughout 2023, and it is uncertain whether we will be able to offset the impact of inflationary pressures in the near term.
Results of Operations
Years Ended December 31, 2022 and 2021
Our financial results for the years ended December 31, 2022 and 2021 are summarized as follows:
| For the years ended December 31, | ||||||||||||||||
| 2022 | 2021 | Change | % Change | |||||||||||||
| Revenues | $ | 486,559 | $ | 15,062 | $ | 471,497 | NM | |||||||||
| Cost of services | 113,195 | 17,275 | 95,920 | NM | ||||||||||||
| Selling, general and administrative | 12,524,258 | 4,505,200 | 8,019,058 | 178.0 | % | |||||||||||
| Research and development | 2,665,427 | - | 2,665,427 | NM | ||||||||||||
| Loss from operations | (14,816,321 | ) | (4,507,413 | ) | (10,308,908 | ) | 228.7 | % | ||||||||
| Other income, net | 879,869 | 2,333,892 | (1,454,023 | ) | (162.3 | )% | ||||||||||
| Net loss | $ | (13,936,452 | ) | $ | (2,173,521 | ) | $ | (11,762,931 | ) | 541.2 | % | |||||
Revenues
Revenues for the years ended December 31, 2022 and 2021 related to our Clinics segment. For the year ended December 31, 2022, revenues increased by approximately $471,000 compared to the year ended December 31, 2021. The increase in revenues is primarily driven by the expansion of our Clinics operations in the U.S. related to the at-home market in New York, NY.
Our Therapeutics segment did not generate any revenues during the years ended December 31, 2022 or 2021.
Cost of Services
Cost of services for the years ended December 31, 2022 and 2021 related to our Clinics segment. For the year ended December 31, 2022, cost of services increased by approximately $96,000 compared to the year ended December 31, 2021. The increase in cost of services was primarily driven by the expansion of our operations in the U.S.
Our Therapeutics segment did not incur any costs of services during the years ended December 31, 2022 and 2021.
52
Selling, General and Administrative
Selling, general and administrative expense increased by approximately $8.0 million, or 178%, for the year ended December 31, 2022 compared to the year ended December 31, 2021. The increase was primarily driven by increases to (i) aggregate costs of approximately $0.9 million in connection with being a public company for the full period in 2022 as compared to the partial period in 2021, and non-recurring corporate communication costs associated with a dissident shareholder campaign, (ii) legal fees of approximately $2.7 million primarily associated with non-recuring costs of a dissident shareholder campaign, litigation settlements, the acquisition of Alpha-5, and increased compliance requirements as a public company, (iii) personnel and third-party contractor costs of approximately $1.6 million related to the hiring of employees and contractors, (iv) insurance of approximately $0.5 million primarily attributable to appropriate directors and officers coverage, (v) bad debt expense of approximately $0.4 million, (vi) Board fees of approximately $0.5 million, (vii) accounting and audit fees of approximately $0.4 million attributable to being a public company for full period in 2022 as compared to the partial period in 2021 and the acquisitions of Alpha-5 and AlloMek, (viii) rent costs of approximately $0.2 million related to our facilities in California and Florida, and (ix) advertising and marketing of approximately $0.4 million related to the Clinics segment.
For the year ended December 31, 2022, approximately $1.9 million of the total $8.0 million increase was attributable to our Clinics segment.
We expect selling, general and administrative expenses to decrease in fiscal year 2023 as we no longer expect to incur non-recurring expenses in connection with acquisitions, the now-resolved dissident shareholder campaign or our Clinics segment.
Research and Development
Research and development for the year ended December 31, 2022 relates activities performed by our Therapeutics segment, and are primarily focused on the development of PAS-001, PAS-002, PAS-003 and PAS-004.
For the year ended December 31, 2022, research and development expenses were $2.7 million. There were no research and development activities during the year ended December 31, 2021. The increase is due to the commencement and expansion of our drug development activities related to our product candidates. The increase was slightly offset by an increase of approximately $0.2 million of grant income from our grant agreement with FightMND, which we became party to in connection with the acquisition of Alpha-5.
We expect research and development expenses to increase in fiscal year 2023 primarily related to manufacturing and clinical development of PAS-004.
Our Clinics segment does not perform any research or development activities.
53
Other Income, Net
For the year ended December 31, 2022, Other income, net decreased by approximately $1.5 million, or 162%, as compared to the year ended December 31, 2021. The decrease was primarily driven by losses of $1.0 million associated with litigation settlements. The decrease was further driven by a $0.5 million decrease in gains associated with changes in the fair value of our warrant liabilities.
Working Capital
| As of December 31, | ||||||||
| 2022 | 2021 | |||||||
| Current assets | $ | 34,076,693 | $ | 53,300,457 | ||||
| Current liabilities | 1,877,634 | 447,280 | ||||||
| Working capital | $ | 32,199,059 | $ | 52,853,177 | ||||
Working capital decreased by $20.6 million between December 31, 2021 and December 31, 2022 due primarily to cash used to fund our $14.8 million loss from operations for the period ended December 31, 2022. Additionally, we paid $3.2 million in cash to repurchase shares of our Common Stock in connection with the Camac Action settlement, and paid $1.7 million in cash in connection with the acquisition of AlloMek.
Liquidity and Capital Resources
| Year Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Net loss | $ | (13,936,452 | ) | $ | (2,173,521 | ) | ||
| Net cash used in operating activities | (14,561,921 | ) | (3,174,058 | ) | ||||
| Net cash used in investing activities | (2,061,546 | ) | (21,503 | ) | ||||
| Net cash (used in) provided by financing activities | (3,206,244 | ) | 55,929,178 | |||||
| Effect of foreign currency translation | 9,900 | (10,561 | ) | |||||
Decrease (increase) in cash and cash equivalents | $ | (19,819,811 | ) | $ | 52,723,056 | |||
The decrease in cash and cash equivalents was primarily attributable to cash used to fund our operations. The decrease in cash and cash equivalents was driven further by the repurchase of shares in connection with Camac Action settlement, and by cash paid in connection with the acquisition of AlloMek.
Liquidity & Capital Resources Outlook
As of December 31, 2022, we had $33.1 million in our operating bank accounts and working capital of $32.2 million. Our major sources of cash have been the proceeds from various private securities offerings, our Initial Public Offering, and the receipt of cash upon the exercise of our outstanding warrants. We are dependent on obtaining additional working capital funding from the sale of equity and/or debt securities in order to continue to execute our development plans and continue operations. Based on the foregoing, management believes that we will have sufficient working capital to meet our liquidity needs through twelve months from the issuance date of the financial statements included in this annual report.
November 2021 Private Placement
On November 24, 2021, issued 8,680,000 shares of our Common Stock (the “PIPE Shares”) and warrants to purchase up to 8,680,000 shares of our Common Stock (“PIPE Warrants”) in a private placement (“November 2021 Private Placement”). The combined purchase price for one PIPE Share and PIPE Warrant was $3.50. The PIPE Warrants are immediately exercisable, expire five years from the date of issuance and have an exercise price of $3.50 per share, subject to adjustment as set forth in the PIPE Warrants. The November 2021 Private Placement resulted in aggregate gross proceeds to us of $30,380,000.
Off-Balance Sheet Arrangements
We did not have any off-balance sheet arrangements as defined in Item 303(a)(4)(ii) of Regulation S-K promulgated under the Exchange Act.
54
Critical Accounting Policies and Estimates
Our critical accounting policies, which include (1) revenue recognition, (2) stock-based compensation and (3) fair value measurements, are more fully described in the notes to our financial statements included in our 10-K for the fiscal year ended December 31, 2022. We believe that the following critical accounting estimates are particularly subject to management’s judgment and could materially affect our financial condition and results of operations:
| ● | Assumptions used in the Black-Scholes pricing model for valuation of stock option awards, such as expected volatility, risk-free interest rate, expected term and expected dividends. |
| ● | Valuation of the liability for Warrants, which requires that we make certain assumptions involving assumptions similar to those described above, as well as to changes in relative fair value. |
| ● | Assumptions used in the valuing of our intangible assets related to our acquisition, and those used in the calculation of the potential earnout. |
For additional information on critical accounting policies and estimates, see Note 2 to the consolidated Financial Statements, “Summary of Significant Accounting Policies and New Accounting Standards,” in Part I, Item 1, of this Annual Report on Form 10-K.
New Accounting Standards
For discussion of new accounting standards, see Note 2 to the consolidated Financial Statements, “Summary of Significant Accounting Policies and New Accounting Standards,” in Part I, Item 1, of this Annual Report on Form 10-K.
Subsequent Events
Nasdaq Deficiency Notice
On January 19, 2023, we received a written notice (the “Notice”) from the Listing Qualifications Department of The Nasdaq Stock Market (“Nasdaq”) indicating that we are not in compliance with the $1.00 minimum bid price requirement set forth in Nasdaq Listing Rule 5550(a)(2) for continued listing on The Nasdaq Capital Market (the “Bid Price Requirement”). The Notice does not result in the immediate delisting of our Common Stock from The Nasdaq Capital Market.
The Nasdaq Listing Rules require listed securities to maintain a minimum bid price of $1.00 per share and, based upon the closing bid price of our Common Stock for 30 consecutive business days prior to the delivery of the Notice, we no longer meet this requirement. The Notice indicated that we will be provided 180 calendar days in which to regain compliance, or until July 18, 2023. If at any time during this period the bid price of our Common Stock closes at or above $1.00 per share for a minimum of ten consecutive business days, the Nasdaq staff (the “Staff”) will provide us with a written confirmation of compliance and the matter will be closed.
Alternatively, if we fail to regain compliance with Rule 5550(a)(2) prior to the expiration of the initial 180 calendar day period, we may be eligible for an additional 180 calendar day compliance period, provided (i) we meet the continued listing requirement for market value of publicly held shares and all other applicable requirements for initial listing on The Nasdaq Capital Market (except for the Bid Price Requirement) and (ii) we provide written notice to Nasdaq of our intention to cure this deficiency during the second compliance period by effecting a reverse stock split, if necessary. In the event we do not regain compliance with Rule 5550(a)(2) prior to the expiration of the initial 180 calendar day period, and if it appears to the Staff that we will not be able to cure the deficiency, or if we are not otherwise eligible, the Staff will provide us with written notification that our securities are subject to delisting from The Nasdaq Capital Market. At that time, we may appeal the delisting determination to a hearings panel.
55
Issuance of Stock Options
On February 24, 2023, we issued stock options under the 2021 Plan to purchase an aggregate of 880,000 shares of Common Stock to certain employees. These stock options had a strike price of $0.491 per share and an expiration term of ten years.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not applicable.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The information called for by Item 8 is included following the “Index to Financial Statements” on page F-1 contained in this Annual Report on Form 10-K.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act and regulations promulgated thereunder) as of December 31, 2022, or the Evaluation Date. Based on such evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that, as of the Evaluation Date, our disclosure controls and procedures were effective.
Management’s Report on Internal Control over Financial Reporting
Our management, under the supervision of the Chief Executive Officer and Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting for our company. Internal control over financial reporting is defined in Rule 13a-15(f) or 15d-15(f) promulgated under the Exchange Act as a process designed by, or under the supervision of, the Company’s principal executive and principal financial officers and effected by the Board, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP and includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of our company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our company’s assets that could have a material effect on the financial statements.
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our internal control over financial reporting as of December 31, 2022. In making this evaluation, our management used the criteria set forth in the Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
56
Based on this evaluation, management concluded that our internal control over financial reporting was effective at a reasonable assurance level as of December 31, 2022 based on those criteria.
This annual report does not include an attestation report of our registered public accounting firm on our internal control over financial reporting due to an exemption established by the JOBS Act for “emerging growth companies.” In addition, we are currently a non-accelerated filer and are therefore not required to provide an attestation report on our internal control over financial reporting until such time as we are an accelerated filer or large accelerated filer.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during the quarter ended December 31, 2022 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
On March 28, 2023, Dr. Yassine Bendiabdallah notified us that he is resigning as Chief Operating Officer and Head of U.K. Clinics, effective as of March 28, 2023.
On March 29, 2023, the Board amended and restated our Bylaws, to provide, among other things, that (i) only the Board, a majority of the members of a committee of the Board, the Chairman of the Board, or the President may call a special meeting of stockholders, (ii) specifying additional information required to be disclosed in any director nomination notice or notices of proposed stockholder business, including additional information regarding related party transactions between director nominees and stockholder associated persons and third-party compensation arrangements involving director nominees, (iii) allowing for the Board, or the holders of the majority of shares represented at a meeting, to postpone or adjourn any annual or special meeting of stockholders, whether or not a quorum is present, and (iv) eliminating the requirement that the list of stockholders be open to examination at meetings of stockholders to conform to recent amendments to the Delaware General Corporation Law.
The foregoing description of our Bylaws is qualified in its entirety by reference to the full text of the Bylaws, which is filed as an exhibit to this Annual Report on Form 10-K, which is incorporated by reference herein.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
57
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Executive Officers, Non-executive employees and Directors
The following table sets forth the name, age as of March 30, 2023, and position of the individuals who serve as directors and executive officers of the Company. The following also includes certain information regarding the individual experience, qualifications, attributes and skills of our directors and executive officers as well as brief statements of those aspects of our directors’ backgrounds that led us to conclude that they are qualified to serve as directors.
| Name | Age | Position | ||
| Executive Officers | ||||
| Dr. Tiago Reis Marques | 46 | Chief Executive Officer and Director | ||
Daniel Schneiderman Non-Employee Directors |
45 | Chief Financial Officer | ||
| Prof. Lawrence Steinman (2)(3) | 75 | Executive Chairman and Co-Founder | ||
| Simon Dumesnil (1)(2)(3) | 46 | Director | ||
| Dr. Emer Leahy (1)(2)(3) | 57 | Director | ||
| Alfred Novak (1) | 75 | Director |
| (1) | Member of the Audit Committee. |
| (2) | Member of the Compensation Committee. |
| (3) | Member of the Nominating and Corporate Governance Committee. |
Executive Officers
Each executive officer serves at the discretion of our Board and holds office until his or her successor is duly elected and qualified or until his or her earlier resignation or removal.
Dr. Tiago Reis Marques (Chief Executive Officer and Director) has served on our Board and as Chief Executive Officer since August 2020. He was a senior clinical fellow at Imperial College London and a lecturer at the IoPPN, King’s College London. IoPPN is ranked second in the world for psychology and psychiatry by US News and Best Global Universities, and is home to one of the world’s largest centers for neuroscience research. Dr. Marques is also a psychiatrist at Maudsley Hospital. His research focuses on topics including the mechanism of action of psychiatric medication and novel treatment targets. During his career, he has obtained multiple awards for his research. Dr. Marques is an author or co-author of more than 100 scientific publications in peer-reviewed journals in psychiatry and neuroscience, has an h-index above 40 and has co-authored international treatment guidelines and written book chapters, including in the leading book in the field, “Neurobiology of Mental Illness.” Dr. Marques received his MD from Coimbra University Medical School in Portugal in 2001 and his PhD from the Kings College Institute of Psychiatry in the UK in 2012. We believe that Dr. Marques is qualified to serve on our Board due to his medical and scientific background.
Daniel Schneiderman (Chief Financial Officer) is a seasoned finance executive with over 20 years of experience in the areas of capital markets and finance operations. Mr. Schneiderman has served as our Chief Financial Officer since October 11, 2022 and as a consultant to the Company from July 1, 2022 through October 10, 2022. Prior to joining the Company, from January 2020 through February 2022 Mr. Schneiderman served as Chief Financial Officer of First Wave BioPharma, Inc. (Nasdaq: FWBI), a clinical stage biopharmaceutical company specializing in the development of targeted, non-systemic therapies for gastrointestinal (GI) diseases. Prior to joining First Wave, from November 2018 through December 2019, Mr. Schneiderman served as Chief Financial Officer of Biophytis SA, (ENXTPA: ALBPS; Nasdaq: BPTS) and its U.S. subsidiary, Biophytis, Inc., a European-based, clinical-stage biotechnology company focused on the development of drug candidates for age-related diseases, with a primary focus on neuromuscular diseases. From February 2012 through August 2018, Mr. Schneiderman served as Vice President of Finance, Controller and Secretary of MetaStat, Inc. (OTCQB: MTST), a publicly traded biotechnology company with a focus on Rx/Dx precision medicine solutions to treat patients with aggressive (metastatic) cancer. From 2008 through February 2012, Mr. Schneiderman was Vice President of Investment Banking at Burnham Hill Partners LLC, a boutique investment bank providing capital raising, advisory and merchant banking services primarily in the healthcare and biotechnology industries. From 2004 through 2008, Mr. Schneiderman served in various roles and increasing responsibilities, including as Vice President of Investment Banking at Burnham Hill Partners, a division of Pali Capital, Inc. Previously, Mr. Schneiderman worked at H.C. Wainwright & Co., Inc. in 2004 as an investment banking analyst. Mr. Schneiderman holds a bachelor’s degree in economics from Tulane University.
58
Non-Employee Directors
Prof. Lawrence Steinman has served on our Board since August 2020. Prior to joining Pasithea, he served on the Board of Centocor from 1989 to 1998, the Board of Neurocine Biosciences from 1997 to 2005, the Board of Atreca (NASDAQ: BCEL) from 2010 to 2019, the Board of BioAtla (NASDAQ: BCAB) from 2016 to the present, the Board of Tolerion from 2013 to the present, the Board of 180 Life Sciences (NASDAQ: ATNF) from 2021 to present, and the Board of Pharnext from 2019 to present. He is currently the George A. Zimmermann Endowed Chair in the Neurology Department at Stanford University and previously served as the Chair of the Interdepartmental Program in Immunology at Stanford University Medical School from 2003 to 2011. He is a member of the National Academy of Medicine and the National Academy of Sciences. He also founded the Steinman Laboratory at Stanford University, which is dedicated to understanding the pathogenesis of autoimmune diseases, particularly multiple sclerosis and neuromyelitis optica. He received the Frederic Sasse Award from the Free University of Berlin in 1994, the Sen. Jacob Javits Award from the U.S. Congress in 1988 and 2002, the John Dystel Prize in 2004 from the National MS Society in the U.S., the Charcot Prize for Lifetime Achievement in Multiple Sclerosis Research in 2011 from the International Federation of MS Societies and the Anthony Cerami Award in Translational Medicine by the Feinstein Institute of Molecular Medicine in 2015. He also received an honorary Ph.D. at the Hasselt University in 2008. He received his BA (physics) from Dartmouth College in 1968 and his MD from Harvard University in 1973. He also completed a fellowship in chemical immunology at the Weizmann Institute (1974 - 1977) and was an intern and resident at Stanford University Medical School. We believe that Prof. Steinman is qualified to serve on our Board due to his extensive background in medicine and his experience as a board member in the life sciences industry.
Simon Dumesnil has served on our Board since April 2021. He is currently a Managing Partner and Director of Dunraven Capital Partners Limited, an investment management advisory company incorporated in the U.K. whose investments are predominately in Eastern European corporate distressed credits and structured products. From 2013 to 2018, Mr. Dumesnil was Managing Director and Head of Structured Financing Group Americas of UBS Securities LLC, where he was responsible for the structured financing trading book in the USA and LATAM and managed a book of financing positions across fixed income products (corporate syndicated and middle-market loans, corporate bonds, real estate loans, CMBS/RMBS/CLO/ABS, LATAM Sovereign). From 2010 to 2013, he was Managing Director and Co-Head Private-Side Structuring Group EMEA of UBS AG., where he was responsible for arranging structured solution transactions and acquisitions for FIG and Special Situation Group (SSG) and also co-headed the illiquid financing business. From 2009 to 2010, Mr. Dumesnil was the Chief Investment Officer Bluestone Capital Management and responsible for investments in distressed assets across Europe. From 2008 to 2009, Mr. Dumesnil was Director of Lehman Brother Holding Inc. and responsible for restructuring and unwinding Lehman Brothers Special Financing Inc. derivative book post-bankruptcy. From 2003 to 2008, Mr. Dumesnil was Director of Lehman Brothers International (Europe). Throughout his career at Dunraven Capital Management, UBS Securities, UBS AG, Bluestone Capital Management and Lehman Brothers, Mr. Dumesnil advised and underwritten corporate risk related to companies across industries or jurisdictions. He has an in-depth knowledge on corporate restructuring and capital structure optimization for companies across their business life cycle. His experience as Chief Investment Officer during the launch and growth phases of a financial services and technology company represents valuable insights for our Company. Mr. Dumesnil attended Cass Business School, where he received his Master of Science in Banking and International Finance and École des Hautes-Études-Commerciales HEC, where he received his Bachelor in Business and Administration, Finance. We believe that Mr. Dumesnil is qualified to serve on our Board due to his management and investment experience.
Dr. Emer Leahy has served on our Board since June 2021. Dr. Leahy received her Ph.D. in neuropharmacology from University College Dublin, Ireland in 1990, and her MBA from Columbia University in 2000. She has been with PsychoGenics Inc., a preclinical CNS service company, since 1999 and is currently serving as its chief executive officer and is responsible for compensation recommendations companywide. Prior to her appointment as the chief executive officer, she was the vice president of business development. Dr. Leahy is also the chief executive officer of PGI Drug Discovery LLC, a company engaged in psychiatric drug discovery with five partnered clinical programs including one in Phase III. Additionally, Dr. Leahy served as a member of both the compensation committee and the audit committee of Bright Minds Biosciences Inc. (NASDAQ: DRUG), a biotech company, until April 2022, and she has served as a member of the Board of Intensity Therapeutics, Inc. since 2016. Dr. Leahy has more than 30 years of experience in drug discovery, clinical development and business development for pharmaceutical and biotechnology companies, including extensive knowledge of technology assessment, licensing, mergers and acquisitions, and strategic planning. She also holds an Adjunct Associate Professor of Neuroscience position at Mount Sinai School of Medicine. Dr. Leahy served on the Emerging Companies Section Governing Board for the Board of the Biotechnology Industry Organization, the Business Review Board for the Alzheimer’s Drug Discovery Foundation, and the Scientific Advisory Board of the International Rett Syndrome Foundation. She also currently serves on the Board of PsychoGenics Inc, the Board of Intensity Therapeutics, and the Board of Trustees of BIONJ. We believe that Dr. Leahy is qualified to serve on our Board due to her extensive pharmaceutical, biotechnology and business background.
59
Alfred Novak has served on our Board since September 2022. Mr. Novak has broad operating experience as a Chief Executive Officer and Chief Financial Officer and has served on the boards of several pharmaceutical and medical device companies. Mr. Novak brings financial acumen and extensive expertise in product development, regulatory approval, commercial activities, and a track record of delivering substantial value for stockholders. Between October 2015 to June 2022, Mr. Novak served as a director of LivaNova Plc (NASDAQ: LIVN), which is a medical device company. From May 2017 to November 2019, Mr. Novak served as a director of Dova Pharmaceuticals, which was sold to Swedish Orphan Biovitrum AB or Sobi™, a company focused on rare diseases, for over $900 million; a director and CEO of Biosense, which was sold to Johnson & Johnson for $400 million; and CFO of Cordis Corporation, which was acquired by Johnson & Johnson for $1.8 billion. He received his MBA from the Wharton School of the University of Pennsylvania with a concentration in Healthcare Administration and a BS from the United States Merchant Marine Academy. We believe Mr. Novak is qualified to serve on our Board due to his extensive experience in product development, the regulatory approval process and commercialization in the pharmaceutical and medical device industries.
Scientific Advisory Board
Professor Charles B. Nemeroff, M.D., Ph.D.
Prof. Charles B. Nemeroff, M.D., Ph.D., is a Professor and Chair of the Department of Psychiatry and Behavioral Sciences at the University of Texas Dell Medical School and Matthew P. Nemeroff Endowed Chair. His research is focused on the pathophysiology of mood and anxiety disorders, and he has published more than 1,100 research reports and reviews. Prof. Nemroff has received numerous research and education awards, including the Kempf Award in Psychobiology, the Samuel Hibbs Award, Research Mentoring Award, Judson Marmot Award and the Vestermark Award from the American Psychiatric Association (APA), the Mood Disorders Award, Bowis Award and Dean Award from the American College of Psychiatrists (ACP) and the Julius Axelrod Award for mentoring from the ACNP. He currently sits on the Scientific Advisory Board of the Brain and Behavioral Research Foundation. Prof. Nemeroff is a member of the National Academy of Medicine. Prof. Nemeroff received his medical degree and doctorate at the University of North Carolina School of Medicine.
Daniel R. Weinberger, M.D.
Dr. Weinberger is Director and CEO of the Lieber Institute for Brain Development at the Johns Hopkins Medical Center and Professor of Psychiatry, Neurology, Neuroscience and Human Genetics at the Johns Hopkins School of Medicine. He was formally Director of the Genes, Cognition, and Psychosis Program of the Intramural Research Program, National Institute of Mental Health, National Institutes of Health in Bethesda, Maryland. He attended college at the Johns Hopkins University and medical school at the University of Pennsylvanian and did residencies in psychiatry at Harvard Medical School and in neurology at George Washington University. He is board certified in both psychiatry and neurology. Dr. Weinberger’s research has focused on brain and genetic mechanisms involved in the pathogenesis and treatment of neuropsychiatric disorders, especially schizophrenia. He was instrumental in focusing research on the role of abnormal brain development as a risk factor for schizophrenia. His has identified a number of specific neural and molecular mechanisms of genetic risk for schizophrenia, and genetic effects that account for variation in specific human cognitive functions and in human temperament. His recent work has focused on genetic and epigenetic regulation of expression in human brain of genes associated with developmental brain disorders. In 2003, Science magazine highlighted the genetic research of his lab as the second biggest scientific breakthrough of the year, second to the origins of the cosmos. He is the recipient of many honors and awards, including the Sarnat International Prize of the National Academy of Medicine, The International Neuroscience Prize of the Gertrud Reemtsma Foundation of the Max Planck Society, the NIH Directors Award, The Roche-Nature Medicine Neuroscience Award, The William K. Warren Medical Research Institute Award, the Adolf Meyer Prize of the American Psychiatric Association, , the Foundation’s Fund Prize from the American Psychiatric Association, and the Lieber Prize of the Brain and Behavior Research Foundation. He is past president of the Society of Biological Psychiatry, past President of the American College of Neuropsychopharmacology and has been elected to the National Academy of Medicine of the National Academy of Sciences.
60
Merit Cudkowicz, M.D.
Dr. Cudkowicz is the Chief of Neurology at Massachusetts General Hospital, Director of the Sean M. Healey & AMG Center for ALS, and the Julieanne Dorn Professor of Neurology at Harvard Medical School. A member of the National Academy of Medicine, Dr. Cudkowicz has been a pioneer in promoting and devising more efficient methods for the development of new therapies for people with neurological disorders such as ALS and is one of the founders and co-directors of the Northeast ALS (NEALS) Consortium, a group of over 130 clinical sites in the United States and Canada dedicated to performing collaborative academic-led clinical trials in ALS. Dr. Cudkowicz is also the Study Chair and Principal Investigator of the HEALEY ALS Platform Trial, a perpetual multi-center, multi-regimen clinical trial evaluating the safety and efficacy of investigational products for the treatment of ALS. Dr. Cudkowicz received the American Academy of Neurology 2009 Sheila Essay ALS award, the 2017 Forbes Norris Award from the International MND Alliance, the 2017 Pinnacle Award from the Boston Chamber of Commerce and the 2019 Ray Adams American Neurological Association Award. She received a B.S. in Chemical Engineering from Massachusetts Institute of Technology, an M.D. from Harvard Medical School and a MSc. in Clinical Epidemiology from Harvard School of Public Health.
Board Composition and Election of Directors
Our Board currently consists of five members. Under our Bylaws, the number of directors who shall constitute the Board shall equal not less than one nor more than ten, as the Board or the majority of our stockholders of record may determine by resolution from time to time.
Board Elections
In accordance with our Bylaws, our stockholders shall elect the directors at our annual meeting of stockholders (except as otherwise provided therein for the filling of vacancies). Each director shall hold office until his or her death, resignation, retirement, removal, or disqualification, or until his or her successor shall have been elected and qualified.
Board Leadership Structure
Our corporate governance guidelines provide that, if the Chairman of the Board is a member of management or does not otherwise qualify as independent, the independent directors of the Board may elect a lead director. The lead director’s responsibilities include, but are not limited to: presiding over all meetings of the Board at which the chairman is not present, including any executive sessions of the independent directors; approving Board meeting schedules and agendas; and acting as the liaison between the independent directors and the Chief Executive Officer and Chairman of the Board. Our corporate governance guidelines further provide the flexibility for our Board to modify our leadership structure in the future as it deems appropriate.
Role of the Board in Risk Oversight
One of the key functions of our Board is informed oversight of our risk management process. Our Board does not have a standing risk management committee, but rather administers this oversight function directly through our Board as a whole, as well as through various standing committees of our Board that address risks inherent in their respective areas of oversight. In particular, our Board is responsible for monitoring and assessing strategic risk exposure and our Audit Committee has the responsibility to consider and discuss our major financial risk exposures and the steps our management has taken to monitor and control these exposures, including guidelines and policies to govern the process by which risk assessment and management is undertaken. Our Audit Committee also monitors compliance with legal and regulatory requirements. Our nominating and corporate governance committee (“Nominating and Corporate Governance Committee”) monitors the effectiveness of our corporate governance practices, including whether they are successful in preventing illegal or improper liability-creating conduct. Our Compensation Committee assesses and monitors whether any of our compensation policies and programs has the potential to encourage excessive risk-taking. While each committee is responsible for evaluating certain risks and overseeing the management of such risks, our entire Board is regularly informed through committee reports about such risks.
61
Board Committees
We currently have three committees of the Board and have adopted charters for such committees: an Audit Committee, a Compensation Committee, and a Nominating and Corporate Governance Committee. The composition and responsibilities of each committee are described below. Members serve on these committees until their resignation or until otherwise determined by our Board. Each committee’s charter is available under the Corporate Governance section of our website at www.pasithea.com. The reference to our website address does not constitute incorporation by reference of the information contained at or available through our website, and you should not consider it to be a part of this 10-K.
Audit Committee. The Audit Committee’s responsibilities include:
| ● | appointing, approving the compensation of, and assessing the independence of our registered public accounting firm; | |
| ● | overseeing the work of our registered public accounting firm, including through the receipt and consideration of reports from such firm; | |
| ● | reviewing and discussing with management and the registered public accounting firm our annual and quarterly financial statements and related disclosures; | |
| ● | coordinating our Board’s oversight of our internal control over financial reporting, disclosure controls and procedures and code of business conduct and ethics; | |
| ● | discussing our risk management policies; | |
| ● | meeting independently with our internal auditing staff, if any, registered public accounting firm and management; | |
| ● | reviewing and approving or ratifying any related person transactions; and | |
| ● | preparing the Audit Committee report required by SEC rules. |
The members of our Audit Committee are Simon Dumesnil (chairperson), Dr. Emer Leahy and Alfred Novak. All members of our Audit Committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC and Nasdaq. Our Board has determined that Simon Dumesnil is an audit committee financial expert as defined under the applicable rules of the SEC and has the requisite financial sophistication as defined under the applicable rules and regulations of Nasdaq. Under the rules of the SEC, members of the Audit Committee must also meet heightened independence standards. Our Board has determined that Simon Dumesnil (chairperson), Dr. Emer Leahy and Alfred Novak are independent within the meaning of the rules and regulations of Nasdaq and Rule 10A-3 under the Exchange Act. Prof. Lawrence Steinman served as a member of our Audit Committee until September 2022.
The Audit Committee operates under a written charter that satisfies the applicable standards of the SEC and Nasdaq.
Compensation Committee. The Compensation Committee’s responsibilities include:
| ● | reviewing and approving, or recommending for approval by the Board, the compensation of our Chief Executive Officer and our other executive officers; | |
| ● | overseeing and administering our cash and equity incentive plans; | |
| ● | reviewing and making recommendations to our Board with respect to director compensation; | |
| ● | reviewing and discussing annually with management our “Compensation Discussion and Analysis,” to the extent required; and | |
| ● | preparing the annual Compensation Committee report required by SEC rules, to the extent required. |
The members of our Compensation Committee are Dr. Emer Leahy (chairperson), Alfred Novak and Simon Dumesnil. Each of the members of our Compensation Committee is independent under the applicable rules and regulations of Nasdaq and is a “non-employee director” as defined in Rule 16b-3 promulgated under the Exchange Act. The Compensation Committee operates under a written charter that satisfies the applicable standards of the SEC and Nasdaq. Prof. Lawrence Steinman served as a member of the Compensation Committee until March 2023 and Mr. Novak joined the Compensation Committee in March 2023.
62
Nominating and Corporate Governance Committee. The Nominating and Corporate Governance Committee’s responsibilities include:
| ● | identifying individuals qualified to become Board members; | |
| ● | recommending to our Board the persons to be nominated for election as directors and to each Board committee; | |
| ● | developing and recommending to our Board corporate governance guidelines, and reviewing and recommending to our Board proposed changes to our corporate governance guidelines from time to time; and | |
| ● | overseeing a periodic evaluation of our Board. |
The members of our Nominating and Corporate Governance Committee are Alfred Novak (chairperson), Dr. Emer Leahy and Simon Dumesnil. Each of the members of our Nominating and Corporate Governance Committee is an independent director under the applicable rules and regulations of Nasdaq relating to Nominating and Corporate Governance Committee independence. The Nominating and Corporate Governance Committee operates under a written charter that satisfies the applicable standards of the SEC and Nasdaq. Prof. Lawrence Steinman was a member of our Nominating and Corporate Governance Committee until March 2023 and Mr. Novak joined the Compensation Committee in March 2023.
Director Independence
Our Board has determined that Simon Dumesnil, Dr. Emer Leahy and Alfred Novak are all “independent” as that term is defined under the rules of The Nasdaq Stock Market LLC. Our Board has determined that due to Dr. Tiago Reis Marques’ employment as an executive officer of the Company, he currently has a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director, such that he is not “independent” as that term is defined under the rules of The Nasdaq Stock Market LLC, or the Nasdaq rules. Our Board has also determined that beginning as of June 21, 2022, due to the Company’s transaction with Alpha-5, Prof. Lawrence Steinman has a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director, such that he is not “independent” as that term is defined under the Nasdaq rules.
Notwithstanding the foregoing, the Board determined, under exceptional and limited circumstances, that Prof. Lawrence Steinman’s membership on the Nominating and Corporate Governance Committee, and the Compensation Committee during the period beginning on June 21, 2022 until March 30, 2023 was required by the best interests of the Company and its stockholders due to his extensive experience with the Company’s operations as a founding member, his prior involvement with our compensation practices and director recruitment process as a member of the Compensation Committee, and the Nominating and Corporate Governance Committee, while the Company searched for and engaged a new, appropriately qualified independent board member to replace him. Further, the Board determined, under exceptional and limited circumstances, that Prof. Lawrence Steinman’s membership on the Audit Committee during the period beginning on June 21, 2022 until September 14, 2022 was required by the best interests of the Company and its stockholders due to his extensive experience with the Company’s operations as a founding member. Upon Mr. Alfred Novak’s appointment to the Board and the Audit Committee in September 2022, the Board decided it was no longer necessary to have Prof. Lawrence Steinman serve on the Audit Committee. Further, after an appropriate period of time to allow for Mr. Alfred Novak to become familiar with the Company’s operations, compensation and recruitment policies, in March 2023 the Board decided it was appropriate to appoint Mr. Novak to the Compensation Committee, and the Nominating and Corporate Governance Committees, making it no longer necessary to have Prof. Lawrence Steinman on these committees.
Compensation Committee Interlocks and Insider Participation
No member of our Compensation Committee is a current or former officer or employee. None of our executive officers served as a director or a member of a Compensation Committee (or other committee serving an equivalent function) of any other entity, one of whose executive officers served as a director or member of our Compensation Committee during the last completed fiscal year.
DELINQUENT SECTION 16(a) REPORTS
Section 16(a) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), requires officers and directors of the Company and persons who beneficially own more than ten percent (10%) of the Common Stock outstanding to file initial statements of beneficial ownership of Common Stock (Form 3) and statements of changes in beneficial ownership of Common Stock (Forms 4 or 5) with the SEC. Officers, directors and greater than 10% stockholders are required by SEC regulation to furnish us with copies of all such forms they file.
On September 6, 2022, Eric Shahinian, on behalf of Camac Capital LLC and certain of its affiliate entities, a 10% owner, filed a Form 4 reporting a purchase of our Common Stock one day late, and on August 26, 2022, Avi Geller, on behalf of Leonite Capital LLC and certain of its affiliated entities, a 10% owner, filed a Form 4 reporting a purchase of our Common Stock three days late. Based solely upon review of Forms 3, 4 and 5 (and amendments thereto) filed electronically with the SEC by our executive officers and directors owning more than 10% of our common stock and upon any written representations received from the executive officers and directors, other than as described above, to our knowledge we believe that all other Section 16(a) filing requirements were met timely in fiscal year 2022.
Corporate Code of Conduct and Ethics
Our Board has adopted a written code of business conduct and ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. Copies of our corporate code of conduct and ethics are available, without charge, upon request in writing to Pasithea Therapeutics Corp., 1111 Lincoln Road, Suite 500, Miami Beach, FL 33139, Attn: Secretary and are posted on the investor relations section of our website, which is located at www.pasithea.com. The inclusion of our website address in this 10-K does not include or incorporate by reference the information on our website into this 10-K. We also intend to disclose any amendments to the Corporate Code of Conduct and Ethics, or any waivers of its requirements, on our website.
63
ITEM 11. EXECUTIVE COMPENSATION
As an emerging growth company under the JOBS Act we have opted to comply with the executive compensation disclosure rules applicable to “smaller reporting companies,” which require compensation disclosure for our principal executive officer and the two most highly compensated executive officers (other than our principal executive officer) serving as executive officers at the end of our most recently completed fiscal year (collectively, our “Named Executive Officers”). This section describes the executive compensation program in place for our Named Executive Officers during the years ended December 31, 2022 and December 31, 2021, who are the individuals who served as our principal executive officer and two most highly compensated executive officers.
This section discusses the material components of the executive compensation program for our executive officers who are named in the “Summary Compensation Table” below and the non-employee members of our Board.
Summary Compensation Table
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) (1) | Non-Equity Incentive Plan Compensation ($) | Non-qualified Deferred Compensation Earnings ($) | All Other Compensation ($) | Total ($) (4) | |||||||||||||||||||||||||||
| Tiago Reis Marques, | 2022 | 450,000 | 89,250 | - | - | - | - | - | 539,250 | |||||||||||||||||||||||||||
| Chief Executive Officer | 2021 | 243,750 | - | 288,000 | 140,141 | - | - | - | 671,891 | |||||||||||||||||||||||||||
| Daniel Schneiderman, | 2022 | 135,205 | (2) | 57,500 | - | 174,498 | - | - | - | 367,203 | ||||||||||||||||||||||||||
| Chief Financial Officer | 2021 | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
| Stanley M. Gloss, | 2022 | 60,000 | - | - | - | - | - | - | 60,000 | |||||||||||||||||||||||||||
| Former Chief Financial Officer (3) | 2021 | 67,500 | - | 60,000 | 284,665 | - | - | - | 412,165 | |||||||||||||||||||||||||||
| (1) | In accordance with SEC rules, the amounts in this column reflect the fair value on the grant date of the option awards granted to the named executive, calculated in accordance with ASC Topic 718. Stock options were valued using the Black-Scholes model. The grant-date fair value does not necessarily reflect the value of shares which may be received in the future with respect to these awards. The grant-date fair value of the stock options in this column is a non-cash expense for the Company that reflects the fair value of the stock options on the grant date and therefore does not affect our cash balance. The fair value of the stock options will likely vary from the actual value the holder receives because the actual value depends on the number of options exercised and the market price of our Common Stock on the date of exercise. For a discussion of the assumptions made in the valuation of the stock options, see Note 4 to this Form 10-K for the year ended December 31, 2022. |
| (2) | Mr. Schneiderman was hired as Chief Financial Officer of the Company on October 11, 2022. Salary for Mr. Schneiderman includes $66,667 paid to Mr. Schneiderman as a consultant to the Company from July 1, 2022 through October 10, 2022. |
| (3) | Mr. Gloss passed away on June 7, 2022. |
| (4) | Dr. Yassine Bendiabdallah was appointed as our Chief Operating Officer and Head of U.K Clinics on November 1, 2021. For the years ended December 31, 2022 and December 31, 2021, Dr. Bendiabdallah received a salary of $120,000 and $20,000, respectively. Dr. Bendiabdallah received no other compensation during either year. Effective November 1, 2021, we entered into a Consulting Agreement with Yassine Bendiabdallah to act as the Head of Pasithea Therapeutic U.K., manage all Pasithea U.K. clinics and aid in our E.U. expansion. The Consulting Agreement provides an annual salary of $120,000 to be paid on a monthly basis, includes three weeks of vacation for each year and provides for reimbursement for all reasonable out-of-pocket expenses incurred in connection with the services provided. The Consulting Agreement continues indefinitely until either party decides to terminate the contract. Dr. Bendiabdallah resigned as our Chief Operating Officer and Head of U.K Clinics on March 28, 2023. |
64
Employment Agreements with our Named Executive Officers
Employment Agreement – Dr. Tiago Reis Marques
On January 1, 2022, we entered into an employment agreement with Dr. Marques. Under the terms of Dr. Marques’ employment agreement, he holds the position of Chief Executive Officer and receives a base salary of $450,000 annually. In addition, Dr. Marques is eligible to receive an annual bonus, with a target amount equal to seventy-five percent (75%) of Dr. Marques’ annual base salary. The actual amount of each bonus will be determined by the sole discretion of our Compensation Committee and will be based upon both the Company’s performance and Dr. Marques’ individual performance. Pursuant to the terms of his employment agreement, Dr. Marques is also eligible to participate in all incentive and deferred compensation programs available to other executives or officers of the Company, and will be eligible to participate in any employee benefit plans and equity plans that we may adopt, which plans may be amended by the Company from time to time in its sole discretion.
Pursuant to Dr. Marques’ employment agreement, Dr. Marques was paid $100,000 as a sign on bonus. We also issued to Dr. Marques stock options to purchase 200,000 shares of Common Stock under our 2021 Incentive Plan, with one-third of the total shares vesting on the 12-month anniversary of the grant date, and the remainder vesting in equal quarterly installments thereinafter. Further, we issued to Dr. Marques Restricted Stock Units exercisable for 200,000 shares of Common Stock, with one-third of the total shares underlying the RSUs vesting upon the 12-month anniversary of the grant date, with the remainder vesting in equal quarterly installments thereafter.
We may terminate Dr. Marques’ employment at any time with or without Cause (as that term is defined in Mr. Marques’ employment agreement) and with or without advance notice to Dr. Marques, and Dr. Marques may terminate his employment at any time for any reason upon providing 90 days’ written notice to the Company.
In the event we terminate Dr. Marques’ employment without Cause, we will pay Dr. Marques the equivalent of 12 months of his base annual salary in effect as of the date of termination, subject to standard payroll deductions and withholdings and Dr. Marques’ executing a release of claims against the Company. If we terminate Dr. Marques’ employment for any other reason, Dr. Marques will receive no compensation other than what he has earned at the time of the termination and he will not be entitled to any severance benefits.
Employment Agreement with Daniel Schneiderman
On October 11, 2022, we entered into an employment agreement with Mr. Schneiderman. Under the terms of Mr. Schneiderman’ employment agreement, he holds the position of Chief Financial Officer and receives a base salary of $330,000 annually. In addition, Mr. Schneiderman is eligible to receive an annual bonus, with a target amount equal to thirty-five percent (35%) of Mr. Schneiderman’s annual base salary. The actual amount of each bonus will be determined by the sole discretion of our Compensation Committee and will be based upon both the Company’s performance and Mr. Schneiderman’ individual performance. Pursuant to the terms of his employment agreement, Mr. Schneiderman is also eligible to participate in all incentive and deferred compensation programs available to other executives or officers of the Company, and will be eligible to participate in any employee benefit plans and equity plans that we may adopt, which plans may be amended by the Company from time to time in its sole discretion.
Pursuant to Mr. Schneiderman’s employment agreement, Mr. Schneiderman was paid $30,000 as a sign on bonus. We also issued to Mr. Schneiderman stock options to purchase 300,000 shares of Common Stock under our 2021 Incentive Plan, with one-third of the total shares vesting on the one year anniversary of the grant date, one-third of the total shares vesting on the two year anniversary of the grant date, and one-third of the total shares vesting on the three year anniversary of the grant date.
We may terminate Mr. Schneiderman’s employment at any time with or without Cause (as that term is defined in Mr. Schneiderman’s employment agreement) and with or without advance notice to Mr. Schneiderman, and Mr. Schneiderman may terminate his employment at any time for any reason upon providing 60 days’ written notice to the Company.
In the event we terminate Mr. Schneiderman’ employment without Cause, we will pay Mr. Schneiderman the equivalent of six months of his base annual salary in effect as of the date of termination, subject to standard payroll deductions and withholdings and Mr. Schneiderman’s executing a release of claims against the Company. His stock options will also accelerate and fully vest on his termination date. If we terminate Mr. Schneiderman’ employment for any other reason, Mr. Schneiderman will receive no compensation other than what he has earned at the time of the termination and he will not be entitled to any severance benefits.
Consulting Agreement with Stanley Gloss
On April 13, 2021, we entered into an agreement with Brio Financial Group, LLC (“Brio”) pursuant to which Stanley M. Gloss served as our Chief Financial Officer and provided certain other specified financial and accounting services typically provided by a chief financial officer (the “Brio Agreement”). The term of the Brio Agreement ran through March 31, 2022. The Company paid a monthly fixed fee of $7,500 during the term of the Brio Agreement. In addition, 25,000 restricted shares of Common Stock were issued to Brio which vested over the 1-year term of the Brio Agreement. Further, the Company issued Stanley M. Gloss stock options to purchase up to 100,000 shares of the Company’s Common Stock, which options vested fully upon execution of the Brio Agreement. As of June 7, 2022, Mr. Gloss no longer provided any services to the Company pursuant to the Brio Agreement.
65
Outstanding Equity Awards at Fiscal Year-End
The following table summarizes, for each of our Named Executive Officers, the number of shares of our Common Stock underlying outstanding stock options held as of December 31, 2022:
| Option Awards | Stock Awards | |||||||||||||||||||||||||
| Name | Grant Date | Number of Shares Underlying Unexercised Options (#) Exercisable | Number of Shares Underlying Unexercised Options (#) Unexercisable | Option Exercise Price ($) | Option Expiration Date | Number of Units of Stock That Have Not Vested | Market Value of Units of Stock That Have Not Vested (3)(4) | |||||||||||||||||||
| Tiago Reis Marques, Chief Executive Officer (1) | 12/20/2021 | 66,667 | 133,333 | $ | 1.44 | 12/20/2031 | 133,333 | $ | 85,600 | |||||||||||||||||
| Daniel Schneiderman, Chief Financial Officer (2) | 10/11/2021 | - | 300,000 | $ | 6.00 | 10/11/2031 | - | - | ||||||||||||||||||
| Stanley M. Gloss, Former Chief Financial Officer | - | - | - | - | - | - | - | |||||||||||||||||||
| (1) | Under the terms of Dr. Marques’ Executive Employment Agreement, on December 20, 2021, he received (i) a grant of 200,000 stock options at an exercise price equal to the closing price of the Company’s Common Stock on the grant date and (ii) a grant of 200,000 restricted stock units (“RSUs”). Dr. Marques’ stock options and RSUs each vest over three years, with one-third vesting 12 months after the grant date, and the remainder vesting in equal tranches quarterly for two years thereafter. |
| (2) | Under the terms of Mr. Schneiderman’s Executive Employment Agreement, on October 11, 2022, he received a grant of 300,000 stock options at an exercise price equal to the closing price of the Company’s Common Stock on the grant date. Mr. Schneiderman’s stock options each vest over three years, with one-third vesting one year after the grant date, one-third vesting two years after the grant date and the one-third vesting three years after the grant date. |
| (3) | The market value of unvested RSUs is based on the closing market price of our Common Stock of $0.642 per share on December 31, 2022. |
| (4) | Dr. Bendiabdallah has not been awarded any options or other units of stock. |
There were no option exercises by our Named Executive Officers during our fiscal years ended December 31, 2022 or 2021.
Incentive Award Plans
2021 Incentive Plan
On July 15, 2021, our Board adopted the 2021 Incentive Plan, which plan was approved by our stockholders on July 15, 2021. Under the 2021 Incentive Plan, we may grant cash and equity incentive awards to eligible service providers in order to attract, motivate and retain the talent for which we compete. The material terms of the 2021 Incentive Plan are summarized below.
Types of Awards. The 2021 Incentive Plan provides for the grant of non-qualified stock options (“NQSOs”), incentive stock options (“ISOs”), restricted stock awards, RSUs, unrestricted stock awards, stock appreciation rights (“SARs”) and other forms of stock-based compensation.
Eligibility and Administration. Employees, officers, consultants, directors, and other service providers of the Company and its affiliates are eligible to receive awards under the 2021 Incentive Plan. The 2021 Incentive Plan is administered by the Board with respect to awards to non-employee directors and by the Compensation Committee with respect to other participants, each of which may delegate its duties and responsibilities to committees of the company’s directors and/or officers (all such bodies and delegates referred to collectively as the plan administrator), subject to certain limitations that may be imposed under Section 16 of the Exchange Act, and/or other applicable law or stock exchange rules, as applicable. The plan administrator has the authority to make all determinations and interpretations under, prescribe all forms for use with, and adopt rules for the administration of, the 2021 Incentive Plan, subject to its express terms and conditions. The plan administrator also sets the terms and conditions of all awards under the 2021 Incentive Plan, including any vesting and vesting acceleration conditions.
66
Share Reserve. Pursuant to the 2021 Incentive Plan, we have reserved 1,280,732 shares of the Common Stock for issuance thereunder, which reserve shall be increased annually beginning on January 1, 2022 and ending on and including January 1, 2031, equal to the lesser of (A) 3% of the aggregate number of shares of Common Stock outstanding on the final day of the immediately preceding calendar year or (B) such smaller number of shares as is determined by our Board. The share reserve is subject to the following adjustments:
| ● | The share limit is increased by the number of shares subject to awards granted that later are forfeited, expire or otherwise terminate without issuance of shares, or that are settled for cash or otherwise do not result in the issuance of shares. | |
| ● | Shares that are withheld upon exercise to pay the exercise price of a stock option or satisfy any tax withholding requirements are added back to the share reserve and again are available for issuance under the 2021 Incentive Plan. |
Awards issued in substitution for awards previously granted by a company that merges with, or is acquired by, the Company do not reduce the share reserve limit under the 2021 Incentive Plan.
Director Compensation. The 2021 Incentive Plan provides for an annual limit on non-employee director compensation of $500,000, increased to $750,000 in the fiscal year of a non-employee director’s initial service as a non-employee member of the Board of the Company. This limit applies to the sum of both equity grants that could be awarded to non-employee directors during a fiscal year (based on their value under ASC Topic 718 on the grant date) and cash compensation, such as cash retainers and meeting fees earned during a fiscal year. Notwithstanding the foregoing, the Board reserves the right to make an exception to these limits due to extraordinary circumstances without the participation of the affected director receiving the additional compensation.
Stock Options. ISOs may be granted only to employees of the Company, or to employees of a parent or subsidiary of the Company, determined as of the date of grant of such options. An ISO granted to a prospective employee upon the condition that such person becomes an employee shall be deemed granted effective on the date such person commences employment. The exercise price of an ISO shall not be less than 100% of the fair market value of the shares covered by the awards on the date of grant of such option or such other price as may be determined pursuant to the Internal Revenue Code of 1986, as amended from time to time (the “Code”). Notwithstanding the foregoing, an ISO may be granted with an exercise price lower than the minimum exercise price set forth above if such award is granted pursuant to an assumption or substitution for another option in a manner that complies with the provisions of Section 424(a) of the Code. Notwithstanding any other provision of the 2021 Incentive Plan to the contrary, no ISO may be granted under the 2021 Incentive Plan after 10 years from the date that the 2021 Incentive Plan was adopted. No ISO shall be exercisable after the expiration of 10 years after the effective date of grant of such award, subject to the following sentence. In the case of an ISO granted to a ten percent stockholder, (i) the exercise price shall not be less than 110% of the fair market value of a share on the date of grant of such ISO, and (ii) the exercise period shall not exceed 5 years from the effective date of grant of such ISO.
Restricted Stock and Restricted Stock Units. The Compensation Committee may award restricted stock and RSUs under the 2021 Incentive Plan. Restricted stock awards consist of shares of stock that are transferred to the participant subject to restrictions that may result in forfeiture if specified vesting conditions are not satisfied. RSU awards result in the transfer of shares of stock to the participant only after specified vesting conditions are satisfied. A holder of restricted stock is treated as a current stockholder and shall be entitled to dividend and voting rights, whereas the holder of a restricted stock unit is treated as a stockholder with respect to the award only when the shares are delivered in the future. RSUs may include dividend equivalents. Specified vesting conditions may include performance goals to be achieved during any performance period and the length of the performance period. The Compensation Committee may, in its discretion, make adjustments to performance goals based on certain changes in the Company’s business operations, corporate or capital structure or other circumstances. When the participant satisfies the conditions of an RSU award, the Company may settle the award (including any related dividend equivalent rights) in shares, cash or other property, as determined by the Compensation Committee, in its sole discretion.
Stock Appreciation Rights. Under the 2021 Incentive Plan, the Compensation Committee may grant SARs to any eligible individual at such time or times, in such amounts, and on such terms and conditions as the Compensation Committee determines. Upon the exercise of a SAR, the recipient will be entitled to receive a payment equal to the fair market value per share of our Common Stock as of the date of exercise, less the base price applicable to the right, multiplied by the number of shares with respect to which such right is exercised. Such payment shall be made in the form of shares (valued at their fair market value on the date of exercise), in cash, or in a combination of cash and shares, subject to applicable tax withholdings. The base price of a SAR must be at least equal to the fair market value per share of our Common Stock as of the date of grant. The requirements for vesting and exercisability may be based on the continuous employment or service of a recipient for a specified time period (or periods) or on the attainment of a specified performance goal (or goals) established by the Compensation Committee. The Compensation Committee may, in its sole discretion, accelerate the vesting or exercisability of any SAR at any time.
67
Other Shares or Share-Based Awards. The Compensation Committee may grant other forms of equity-based or equity-related awards other than stock options, restricted stock or restricted stock units. The terms and conditions of each stock-based award shall be determined by the Compensation Committee.
Clawback Rights. Awards granted under the 2021 Incentive Plan will be subject to recoupment or clawback under the Company’s clawback policy or applicable law, both as in effect from time to time.
Sale of the Company. Awards granted under the 2021 Incentive Plan do not automatically accelerate and vest, become exercisable (with respect to stock options), or have performance targets deemed earned at target level if there is a sale of the Company. The Company does not use a “liberal” definition of change in control as defined in Institutional Shareholder Services’ proxy voting guidelines. The 2021 Incentive Plan provides flexibility to the Compensation Committee to determine how to adjust awards at the time of a sale of the Company.
No Repricing. The 2021 Incentive Plan prohibits the amendment of the terms of any outstanding award, and any other action taken in a manner to achieve (i) the reduction of the exercise price of NQSOs, ISOs or stock appreciation rights (collectively, “Stock Rights”); (ii) the cancellation of outstanding Stock Rights in exchange for cash or other awards with an exercise price that is less than the exercise price or base price of the original award; (iii) the cancellation of outstanding Stock Rights with an exercise price or base price that is less than the then current fair market value of a share of Common Stock in exchange for other awards, cash or other property; or (iv) otherwise effect a transaction that would be considered a “repricing” for the purposes of the stockholder approval rules of the applicable securities exchange or inter-dealer quotation system on which the Common Stock is listed or quoted without stockholder approval.
Transferability of Awards. Except as described below, awards under the 2021 Incentive Plan generally are not transferable by the recipient other than by will or the laws of descent and distribution. Any amounts payable or shares issuable pursuant to an award generally will be paid only to the recipient or the recipient’s beneficiary or representative. The Compensation Committee has discretion, however, to permit certain transfer of awards to other persons or entities.
Adjustments. As is customary in incentive plans of this nature, each share limit and the number and kind of shares available under the 2021 Incentive Plan and any outstanding awards, as well as the exercise price or base price of awards, and performance targets under certain types of performance-based awards, are subject to adjustment in the event of certain reorganizations, mergers, combinations, recapitalizations, stock splits, stock dividends, or other similar events that change the number or kind of shares outstanding, and extraordinary dividends or distributions of property to the stockholders.
Amendment and Termination. The Board may amend, modify or terminate the 2021 Incentive Plan without stockholder approval, except that stockholder approval must be obtained for any amendment that, in the reasonable opinion of the Board or the Compensation Committee, constitute a material change requiring stockholder approval under applicable laws, policies or regulations or the applicable listing or other requirements of a stock exchange on which shares of Common Stock are then listed. The 2021 Incentive Plan will terminate upon the earliest of (1) termination of the 2021 Incentive Plan by the Board, or (2) the tenth anniversary of the Board adoption of the 2021 Incentive Plan. Awards outstanding upon expiration of the 2021 Incentive Plan shall remain in effect until they have been exercised or terminated, or have expired.
Indemnification Agreements
We have entered into indemnification agreements with each of our directors and executive officers. These agreements, among other things, require us or will require us to indemnify each director and executive officer to the fullest extent permitted by Delaware law, including indemnification of expenses such as attorneys’ fees, judgments, fines and settlement amounts incurred by the director or executive officer in any action or proceeding, including any action or proceeding by or in right of us, arising out of the person’s services as a director or executive officer. For further information, see “Description of Capital Stock-Limitations on Liability and Indemnification Matters.”
68
Policies and Procedures for Related Person Transactions
Our Board has adopted a written related person transaction policy, setting forth the policies and procedures for the review and approval or ratification of related person transactions. This policy covers, with certain exceptions as set forth in Item 404 of Regulation S-K under the Securities Act, any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, in which we were or are to be a participant, where the amount involved will be the lesser of $120,000 or 1% of assets the average of our total assets at year-end for the last two completed fiscal years, in any fiscal year and a related person had, has or will have a direct or indirect material interest, including without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person. In reviewing and approving any such transactions, our Audit Committee is tasked to consider all relevant facts and circumstances, including, but not limited to (i) whether the transaction is on terms comparable to those that could be obtained in an arm’s length transaction with an unrelated party; (ii) the extent of the related person’s interest in the transaction; (iii) the benefits to the Company; (iv) the impact on a director’s independence in the event the related person is a director, an immediately family member of a director or an entity in which a director is a partner, stockholder or executive officer; (v) the availability of other sources for comparable products or services; (vi) the terms of the transaction; and (vii) the terms available to unrelated third parties.
All related-party transactions may only be consummated if our Audit Committee has approved or ratified such transaction in accordance with the guidelines set forth in the policy. Any member of the Audit Committee who is a related person with respect to a transaction under review will not be permitted to participate in the deliberations or vote respecting approval or ratification of the transaction. However, such director may be counted in determining the presence of a quorum at a meeting of the Audit Committee that considers the transaction.
Limitations on Liability and Indemnification Matters
Our Certificate of Incorporation limits our directors’ liability to the fullest extent permitted under Delaware law, which prohibits our Certificate of Incorporation from limiting the liability of our directors for the following:
| ● | any breach of the director’s duty of loyalty to us or our stockholders; | |
| ● | acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law; | |
| ● | unlawful payment of dividends or unlawful stock repurchases or redemptions; or | |
| ● | any transaction from which the director derived an improper personal benefit. |
If Delaware law is amended to authorize corporate action further eliminating or limiting the personal liability of a director, then the liability of our directors will be eliminated or limited to the fullest extent permitted by Delaware law, as so amended.
Our Bylaws provide that we indemnify our directors and officers to the fullest extent permitted under Delaware law and that we shall have the power to indemnify our employees and agents to the fullest extent permitted by law. Our Bylaws also permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in this capacity, regardless of whether we would have the power to indemnify such person against such expense, liability or loss under the DGCL.
69
We have entered into indemnification agreements with our directors and executive officers, in addition to indemnification provided for in our Bylaws. These agreements, among other things, provide for indemnification of our directors and executive officers for expenses, judgments, fines and settlement amounts incurred by such persons in any action or proceeding arising out of this person’s services as a director or executive officer or at our request. We believe that these provisions in our Certificate of Incorporation and Bylaws and indemnification agreements are necessary to attract and retain qualified persons as directors and executive officers.
The above description of the limitation of liability and indemnification provisions of our Certificate of Incorporation, our Bylaws and our indemnification agreements is not complete and is qualified in its entirety by reference to these documents, each of which is filed as an exhibit to this Form 10-K.
The limitation of liability and indemnification provisions in our Certificate of Incorporation and Bylaws may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might benefit us and our stockholders. A stockholder’s investment may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
Insofar as indemnification for liabilities under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. There is no pending litigation or proceeding naming any of our directors or officers as to which indemnification is being sought, nor are we aware of any pending or threatened litigation that may result in claims for indemnification by any director or officer.
Director Compensation
The following table sets forth for each non-employee director that served as a director during the year ended December 31, 2022 certain information concerning his or her compensation for the year ended December 31, 2022:
Year Ended December 31, 2022
| Name | Fees Earned or Paid in Cash ($) | Stock Awards ($) | Option Awards ($) (1) | Non-equity Incentive Plan Compensation ($) | Nonqualified Deferred Compensation Earnings ($) | All Other Compensation ($) | Total ($)(2) | |||||||||||||||||||||
| Prof. Lawrence Steinman | 160,000 | - | - | - | - | 90,000 | (5) | 250,000 | ||||||||||||||||||||
| Simon Dumesnil | 60,000 | (3) | - | - | - | - | - | 60,000 | ||||||||||||||||||||
| Dr. Emer Leahy | 60,000 | (3) | - | - | - | - | - | 60,000 | ||||||||||||||||||||
| Alfred Novak | 14,658 | (4) | - | 48,933 | - | - | - | 63,591 | ||||||||||||||||||||
| (1) | In accordance with SEC rules, the amounts in this column reflect the fair value on the grant date of the option awards granted to the named executive, calculated in accordance with ASC Topic 718. Stock options were valued using the Black-Scholes model. The grant-date fair value does not necessarily reflect the value of shares which may be received in the future with respect to these awards. The grant-date fair value of the stock options in this column is a non-cash expense for the Company that reflects the fair value of the stock options on the grant date and therefore does not affect our cash balance. The fair value of the stock options will likely vary from the actual value the holder receives because the actual value depends on the number of options exercised and the market price of our Common Stock on the date of exercise. For a discussion of the assumptions made in the valuation of the stock options, see Note 5 (Stockholders’ Equity) to our financial statements, which are included in this 10-K. |
| (2) | All directors receive reimbursement for reasonable out of pocket expenses in attending Board meetings and for participating in our business. |
| (3) | Amount includes (i) $45,000 in fees paid during fiscal year 2021 for services rendered during fiscal year 2022 and (ii) $15,000 in fees paid during fiscal year 2023 for services rendered during fiscal year 2022. |
| (4) | Amount includes $7,537 in fees paid during fiscal year 2023 for services rendered during fiscal year 2022. |
| (5) | Amount received for consulting services rendered to the Company during fiscal year 2022. |
70
Compensation Policy for Non-Employee Directors.
The material terms of the non-employee director compensation program, as it is currently contemplated, are summarized below.
The non-employee director compensation program provides for annual retainer fees and/or long-term equity awards for our non-employee directors. Each non-employee director is eligible to receive an annual retainer of $50,000 plus an additional $10,000 for each Board committee that he or she chairs. A non-employee director serving as Chairman of the Board is eligible to receive an additional annual retainer of $100,000. Additionally, upon joining the Board, Non-employee directors are eligible to receive stock options to purchase 100,000 shares of Common Stock, with 50% of the shares subject to the options vesting after the first year of service and 50% vesting after the second year.
Compensation under our non-employee director compensation policy is subject to the annual limits on non-employee director compensation set forth in the 2021 Incentive Plan, as described above. Our Board or an authorized committee may modify the non-employee director compensation program from time to time in the exercise of its business judgment, taking into account such factors, circumstances and considerations as it shall deem relevant from time to time, subject to the annual limit on non-employee director compensation set forth in the 2021 Incentive Plan. As provided in the 2021 Incentive Plan, our Board or its authorized committee may make exceptions to this limit for individual non-employee directors in extraordinary circumstances, as the Board or its authorized committee may determine in its discretion.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Security Ownership of Certain Beneficial Holders and Management
The following table sets forth information with respect to the beneficial ownership of our Common Stock as of March 27, 2023 by:
| ● | each of our Named Executive Officers; | |
| ● | each of our directors; and | |
| ● | all of our executive officers and directors as a group. |
71
The number of shares beneficially owned by each stockholder is determined in accordance with the rules issued by the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under these rules, beneficial ownership includes any shares as to which the individual or entity has sole or shared voting power or investment power, which includes the power to dispose of or to direct the disposition of such security. Except as indicated in the footnotes below, we believe, based on the information furnished to us, that the individuals and entities named in the table below have sole voting and investment power with respect to all shares of Common Stock beneficially owned by them, subject to any community property laws.
Percentage ownership of our Common Stock is based on 26,126,740 shares of Common Stock outstanding as of March 27, 2023. In computing the number of shares beneficially owned by an individual or entity and the percentage ownership of that person, shares of Common Stock subject to options, restricted units, warrants or other rights held by such person that are currently exercisable or will become exercisable within 60 days of March 27, 2023 are considered outstanding, although these shares are not considered outstanding for purposes of computing the percentage ownership of any other person.
To calculate a stockholder’s percentage of beneficial ownership of Common Stock, we must include in the numerator and denominator those shares of Common Stock, as well as those shares of Common Stock underlying options, warrants and convertible securities, that such stockholder is considered to beneficially own. Shares of Common Stock, and Common Stock underlying options, warrants and convertible securities, held by other stockholders, however, are disregarded in this calculation. Therefore, the denominator used in calculating beneficial ownership of each of the stockholders may be different.
Unless otherwise indicated, the address of each beneficial owner listed below is c/o Pasithea Therapeutics Corp., 1111 Lincoln Road, Suite 500, Miami Beach, FL 33139. To our knowledge, there is no arrangement, including any pledge by any person of securities of the Company, the operation of which may at a subsequent date result in a change in control of the Company.
| Beneficial Ownership | ||||||||
| Common Stock | ||||||||
| Name of Beneficial Owner | Shares (1) | %(2) | ||||||
| 5% or Greater Stockholders | ||||||||
| PD Joint Holdings, LLC (3) | 3,408,696 | 12.7 | % | |||||
| Named Executive Officers and Directors: | ||||||||
| Dr. Tiago Reis Marques (4) | 776,668 | 3.0 | % | |||||
| Daniel Schneiderman (5) | - | * | ||||||
| Prof. Lawrence Steinman (6) | 1,547,174 | 5.9 | % | |||||
| Dr. Emer Leahy (7) | 50,000 | * | ||||||
| Simon Dumesnil (8) | 100,000 | * | ||||||
| Alfred Novak (9) | - | * | ||||||
| Yassine Bendiabdallah (10) | 300,000 | 1.2 | % | |||||
| All Directors and Officers as a group (7 persons) | 2,773,842 | 10.4 | % | |||||
* Less than 1%.
| (1) | Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. All entries exclude beneficial ownership of shares issuable pursuant to warrants, options or other derivative securities that have not vested or that are not otherwise exercisable as of the date hereof or which will not become vested or exercisable within 60 days. |
| (2) | Percentages are rounded to nearest tenth of a percent. Percentages are based on 26,126,740 shares of Common Stock outstanding as of March 27, 2023. Warrants, stock options or other derivative securities that are presently exercisable or exercisable within 60 days are deemed to be beneficially owned by the person holding such securities for the purpose of computing the percentage ownership of that person, but are not treated as outstanding for the purpose of computing the percentage of any other person. |
72
| (3) | Consists of (i) 2,608,696 shares of Common Stock and (ii) 800,000 shares of Common Stock issuable upon the exercise of a warrant held directly by PD Joint Holdings, LLC Series 2016-A. All share information is based on information disclosed in a statement on Schedule 13G filed with the SEC on February 15, 2023 on behalf of Paul B. Manning, Bradford Manning, PD Joint Holdings, LLC, Series 2016-A, and Tiger Lily Capital, LLC. The business address for each person and entity named in this footnote is 200 Garrett Street, Suite S, Charlottesville, Virginia 22902. |
| (4) | Includes (i) 683,334 shares of Common Stock and (ii) 83,334 shares of Common Stock issuable upon exercise of vested stock options. Excludes (i) 116,666 unvested options and (ii) 116,666 unvested restricted stock units. |
| (5) | Excludes 300,000 unvested stock options. |
| (6) | Includes (i) 1,297,174 shares of Common Stock, (ii) 200,000 shares of Common Stock issuable upon exercise of warrants, and (iii) 50,000 shares of Common Stock issuable upon exercise of vested stock options. Excludes 50,000 unvested stock options. |
| (7) | Includes 50,000 shares of Common Stock issuable upon exercise of vested stock options. Excludes 50,000 unvested stock options. |
| (8) | Includes (i) 50,000 shares of Common Stock and (ii) 50,000 shares of Common Stock issuable upon exercise of vested stock options. Excludes 50,000 unvested stock options. |
| (9) | Excludes 100,000 unvested stock options. |
| (10) | Includes 300,000 shares of Common Stock. |
Securities Authorized for Issuance Under Existing Equity Compensation Plans
The following table summarizes certain information regarding our equity compensation plans as of December 31, 2022:
| Plan Category | Number
of to
be Issued Exercise of Outstanding | Weighted-Average Exercise Outstanding Options (2) | Number
of Remaining Future Issuance Plans (Excluding Securities Column (a)) (3) | |||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by security holders (1) | 1,500,000 | $ | 2.28 | 466,483 | ||||||||
| Equity compensation plans not approved by security holders | - | $ | - | - | ||||||||
| Total | 1,500,000 | $ | 2.28 | 466,483 | ||||||||
| (1) | Consists of 1,300,000 stock options and 200,000 restricted stock units under the 2021 Incentive Plan. For a description of the 2021 Plan, see Note 4 to our Consolidated Financial Statements included in this 10-K for the year ended December 31, 2022. |
| (2) | The weighted average exercise price does not take into account outstanding restricted stock units, which have no exercise price. |
| (3) | The number of shares of Common Stock available for grant and issuance under the 2021 Plan is subject to an automatic annual increase on January 1 of each year beginning on January 1, 2022 by an amount equal to 3% of the total number of shares of Common Stock outstanding on December 31 of the preceding calendar year. |
73
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Transactions with Related Persons
Except as set out below, as of January 1, 2021, there have been no transactions, or currently proposed transactions, in which we were or are to be a participant and the amount involved exceeds the lesser of $120,000 or one percent of the average of our total assets at year-end for the last two completed fiscal years, and in which any of the following persons had or will have a direct or indirect material interest:
| ● | any director or executive officer of our company; | |
| ● | any person who beneficially owns, directly or indirectly, shares carrying more than 5% of the voting rights attached to our outstanding shares of Common Stock; | |
| ● | any promoters and control persons; and | |
| ● | any member of the immediate family (including spouse, parents, children, siblings and in laws) of any of the foregoing persons. |
Pursuant to our Audit Committee charter adopted in 2021, the Audit Committee is responsible for reviewing and approving, prior to our entry into any such transaction, all transactions in which we are a participant and in which any parties related to us have or will have a direct or indirect material interest.
The following includes a summary of transactions since January 1, 2021 to which we have been a party in which the amount involved will be the lesser of $120,000 or 1% of our assets, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than 5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest, other than equity and other compensation, termination, change in control and other arrangements, which are described under “Executive and Director Compensation.” We also describe below certain other transactions with our directors, executive officers and stockholders.
Related Party Transactions
Alpha-5 integrin, LLC
On June 21, 2022, we entered into the Alpha-5 Agreement with the Alpha-5 Sellers, pursuant to which the Alpha-5 Sellers sold all of the issued and outstanding equity of Alpha-5 to the Company. Alpha-5 is a preclinical-stage company developing a monoclonal antibody (mAbs) for the treatment of ALS and other neuroinflammatory disorders, such as Multiple Sclerosis. In connection with the transaction, we issued to the Alpha-5 Sellers 3,260,870 shares of our Common Stock, which had a market value of $1.01 million on the date of the transaction, and warrants exercisable for 1,000,000 shares of Common Stock at an exercise price of $1.88 per share, expiring five years from the acquisition date, the aggregate fair value of which was $0.4 million at the date of acquisition.
Prof. Lawrence Steinman, our Executive Chairman and Co-Founder, was a 20% owner of Alpha-5 at the time of the transaction.
Zen Healthcare
During the year ended December 31, 2020, we entered into a Collaboration Agreement, as amended and restated on August 4, 2021 (the “Zen Knightsbridge Collaboration Agreement”) with Purecare Limited (“Purecare”), a company that operates a health clinic known as Zen Knightsbridge Clinic (the “Zen Knightsbridge Clinic”), whereby both parties agreed to collaborate on the provision of treatments at Purecare’s London based clinic. Additionally, during the year ended December 31, 2020, we entered into a Collaboration Agreement, as amended and restated on August 4, 2021 (the “Zen Baker Street Collaboration Agreement”) with Portman Health Ltd (“Portman”), a company that operates a health clinic known as Zen Baker Street Clinic (the “Zen Baker Street Clinic”).
Our former Chief Operating Officer, Head of UK Clinics, Dr. Yassine Bendiabdallah, is a co-founder, current managing director, and 25% shareholder of Purecare. Dr. Bendiabdallah is also a co-founder and 16.25% shareholder of Portman.
PsychoGenics, Inc.
We are currently negotiating with PsychoGenics, Inc. (“PsychoGenics”) for the conduct of one of our preclinical studies. PsychoGenics is a CMO with extensive experience running studies like the one we plan on conducting. Pursuant to the proposed transaction, we anticipate aggregate payments to PsychoGenics may total approximately $0.3 million over the term of the contract.
Dr. Emer Leahy, a member of our Board, is the current Chief Executive Officer and a less than 5% owner of PsychoGenics.
74
Brio Financial Group
On April 13, 2021, we entered into the Brio Agreement pursuant to which Brio provided Stanley M. Gloss to serve as our Chief Financial Officer and also provided certain other specified financial and accounting services typically provided by a chief financial officer. The initial term of the Brio Agreement ran through March 31, 2022. The Company paid a monthly fixed fee of $7,500 during the term of the Brio Agreement. In addition, 25,000 restricted shares of Common Stock were issued to Brio which vested over the 1-year term of the Brio Agreement. Further, the Company issued Stanley M. Gloss stock options to purchase up to 100,000 shares of Common Stock, which options vested fully upon execution of the Brio Agreement and had an exercise price of $5.00 per share. As of June 7, 2022, Mr. Gloss no longer provided any services to the Company pursuant to the Brio Agreement.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The Board of the Company has appointed Marcum LLP as our independent registered public accounting firm (the “Independent Auditor”) for the fiscal year ended December 31, 2022. The following table sets forth the fees billed to the Company for professional services rendered by Marcum LLP for the years ended December 31, 2022 and December 31, 2021:
| Year Ended December 31, | ||||||||
| Services: | 2022 | 2021 | ||||||
| Audit Fees (1) | $ | 295,546 | $ | 179,347 | ||||
| Audit-Related Fees (2) | 51,034 | 16,480 | ||||||
| Tax Fees (3) | - | - | ||||||
| All Other Fees | - | - | ||||||
| Total fees | $ | 346,580 | $ | 195,827 | ||||
| (1) | Audit fees consisted of audit work performed in the preparation of financial statements, as well as work generally only the independent registered public accounting firm can reasonably be expected to provide, such as statutory audits. |
| (2) | Audit related fees consisted principally of procedures related to regulatory filings in 2022 and 2021. |
| (3) | The tax fees were paid for reviewing various tax related matters. |
Policy on Audit Committee Pre-Approval of Audit and Permissible Non-audit Services of Independent Public Accountant
Consistent with SEC policies regarding auditor independence, the Audit Committee has responsibility for appointing, setting compensation and overseeing the work of our independent registered public accounting firm. In recognition of this responsibility, the Audit Committee has established a policy to pre-approve all audit and permissible non-audit services provided by our independent registered public accounting firm.
75
Prior to engagement of an independent registered public accounting firm for the next year’s audit, management will submit an aggregate of services expected to be rendered during that year for each of four categories of services to the Audit Committee for approval.
| 1. | Audit services include audit work performed in the preparation of financial statements, as well as work that generally only an independent registered public accounting firm can reasonably be expected to provide, including comfort letters, statutory audits, and attest services and consultation regarding financial accounting and/or reporting standards. |
| 2. | Audit-Related services are for assurance and related services that are traditionally performed by an independent registered public accounting firm, including due diligence related to mergers and acquisitions, employee benefit plan audits, and special procedures required to meet certain regulatory requirements. |
| 3. | Tax services include all services performed by an independent registered public accounting firm’s tax personnel except those services specifically related to the audit of the financial statements, and includes fees in the areas of tax compliance, tax planning, and tax advice. |
| 4. | Other Fees are those associated with services not captured in the other categories. The Company generally does not request such services from our independent registered public accounting firm. |
Prior to engagement, the Audit Committee pre-approves these services by category of service. The fees are budgeted and the Audit Committee requires our independent registered public accounting firm and management to report actual fees versus the budget periodically throughout the year by category of service. During the year, circumstances may arise when it may become necessary to engage our independent registered public accounting firm for additional services not contemplated in the original pre-approval. In those instances, the Audit Committee requires specific pre-approval before engaging our independent registered public accounting firm.
The Audit Committee may delegate pre-approval authority to one or more of its members. The member to whom such authority is delegated must report, for informational purposes only, any pre-approval decisions to the Audit Committee at its next scheduled meeting.
All services rendered by Marcum after our initial public offering in our fiscal years ended December 31, 2022 and 2021 were pre-approved by our Audit Committee.
76
PART IV
ITEM 15. EXHIBIT AND FINANCIAL STATEMENT SCHEDULES
| a) | Financial Statements |
Our consolidated financial statements are set forth in Part II, Item 8 of this 10-K and are incorporated herein by reference.
| b) | Financial Statement Schedules |
No financial statement schedules have been filed as part of this 10-K because they are not applicable or are not required or because the information is otherwise included herein.
| c) | Exhibits required by Regulation S-K |
77
| * | Filed herewith. |
| ** | Furnished herewith. |
| + | Indicates a management contract or any compensatory plan, contract or arrangement. |
ITEM 16. FORM 10-K SUMMARY
Not applicable.
78
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
PASITHEA THERAPEUTICS CORP.
| By: | /s/ Dr. Tiago Reis Marques | |
| Dr. Tiago Res Marques | ||
| Chief Executive Officer and Director (Principal Executive Officer) |
||
Date: March 30, 2023 |
||
| By: | /s/ Daniel Schneiderman | |
| Daniel Schneiderman | ||
Chief Financial Officer (Principal Financial and Accounting Officer) |
||
Date: March 30, 2023 |
||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Dr. Tiago Reis Marques | Chief Executive Officer and Director | March 30, 2023 | ||
| Dr. Tiago Reis Marques | (Principal executive officer) | |||
| /s/ Daniel Schneiderman | Chief Financial Officer | March 30, 2023 | ||
| Daniel Schneiderman | (Principal financial and accounting officer) | |||
| /s/ Prof. Lawrence Steinman | Director | March 30, 2023 | ||
| Prof. Lawrence Steinman | ||||
| /s/ Simon Dumesnil | Director | March 30, 2023 | ||
| Simon Dumesnil | ||||
| /s/ Dr. Emer Leahy | Director | March 30, 2023 | ||
| Dr. Emer Leahy | ||||
| /s/ Alfred Novak | Director | March 30, 2023 | ||
| Alfred Novak |
79
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
PASITHEA THERAPEUTICS CORP.
CONSOLIDATED FINANCIAL STATEMENTS
TABLE OF CONTENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of
Pasithea Therapeutics Corp.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Pasithea Therapeutics Corp. (the “Company”) as of December 31, 2022 and 2021, the related consolidated statements of operations and comprehensive loss, changes in stockholders’ equity, and cash flows for each of the two years in the period ended December 31, 2022, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2022, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
We have served as the Company’s auditor since 2021.
March 30, 2023
F-2
PASITHEA THERAPEUTICS CORP.
CONSOLIDATED BALANCE SHEETS
| December 31, 2022 | December 31, 2021 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | $ | ||||||
| Prepaid expenses | ||||||||
| Other receivables | ||||||||
| Total current assets | ||||||||
| Property and equipment, net | ||||||||
| Right of use asset-operating lease | ||||||||
| Intangibles, net | ||||||||
| Goodwill | ||||||||
| Total assets | $ | $ | ||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable and accrued liabilities | $ | $ | ||||||
| Lease liability - short term position | ||||||||
| Total current liabilities | ||||||||
| Non-current liabilities | ||||||||
| Lease liability | ||||||||
| Warrant liabilities | ||||||||
| Total non-current liabilities | ||||||||
| Total liabilities | ||||||||
| Commitments and Contingencies (Note 15) | ||||||||
| Stockholders’ equity: | ||||||||
| Preferred stock, par value $ | ||||||||
| Common stock, par value $ | ||||||||
| Additional paid-in capital | ||||||||
| Accumulated other comprehensive income (loss) | ( | ) | ( | ) | ||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Total stockholders’ equity | ||||||||
| Total liabilities and stockholders’ equity | $ | $ | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
PASITHEA THERAPEUTICS CORP.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
| For the Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Revenues | $ | $ | ||||||
| Cost of services | ||||||||
| Gross margin | ( | ) | ||||||
| Operating expenses: | ||||||||
| Selling, general and administrative | $ | $ | ||||||
| Research and development | ||||||||
| Loss from operations | ( | ) | ( | ) | ||||
| Other income (expense): | ||||||||
| Change in fair value of warrant liabilities | ||||||||
| Interest expense | ( | ) | ( | ) | ||||
| Gain on forgiveness of accounts payable | ||||||||
| Litigation settlements | ( | ) | ||||||
| Other income, net | ||||||||
| Loss before income taxes | ( | ) | ( | ) | ||||
| Provision for income taxes | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| $ | ( | ) | $ | ( | ) | |||
| Comprehensive loss: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Foreign currency translation | ( | ) | ||||||
| Comprehensive loss | $ | ( | ) | $ | ( | ) | ||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
PASITHEA THERAPEUTICS CORP.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
| Common Stock | Additional Paid-in | Accumulated Other Comprehensive | Accumulated | Total Stockholders’ | ||||||||||||||||||||
| Shares | Amount | Capital | Loss | Deficit | Equity | |||||||||||||||||||
| Balance at December 31, 2020 | $ | $ | $ | $ | ( | ) | $ | |||||||||||||||||
| Stock-based compensation | - | |||||||||||||||||||||||
| Issuance of shares for cash | ||||||||||||||||||||||||
| Issuance of shares for services | ||||||||||||||||||||||||
| Share adjustment (Note 8) | ||||||||||||||||||||||||
| Issuance of public warrants | - | ( | ) | ( | ) | |||||||||||||||||||
| Issuance of representatives warrants | - | ( | ) | ( | ) | |||||||||||||||||||
| Sale of Units, net of underwriting discounts and offering costs | ||||||||||||||||||||||||
| Sale of common stock and warrants, net of fees and costs | - | - | ||||||||||||||||||||||
| Exercise of warrants for cash | - | - | ||||||||||||||||||||||
| Foreign currency translation | - | ( | ) | ( | ) | |||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||
| Balance at December 31, 2021 | $ | $ | $ | ( | ) | $ | ( | ) | $ | |||||||||||||||
| Stock-based compensation expense | - | |||||||||||||||||||||||
| Shares issued for services | ||||||||||||||||||||||||
| Common shares and warrants issued for acquisition | ||||||||||||||||||||||||
| Common shares and warrants issued for acquisition of intangible assets | - | - | ||||||||||||||||||||||
| Shares repurchased in litigation settlement | ( | ) | ( | ) | - | - | ( | ) | ( | ) | ||||||||||||||
| Foreign currency translation | - | |||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||
| Balance at December 31, 2022 | $ | $ | $ | ( | ) | $ | ( | ) | $ | |||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
PASITHEA THERAPEUTICS CORP.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation | ||||||||
| Stock-based compensation | ||||||||
| Shares issued for services | ||||||||
| Change in fair value of warrant liabilities | ( | ) | ( | ) | ||||
| Amortization expense | ||||||||
| Bad debt expense | ||||||||
| Non-cash lease expense | ||||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses | ( | ) | ( | ) | ||||
| Other current assets | ( | ) | ||||||
| Accounts payable and accrued liabilities | ||||||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Purchase of property and equipment | ( | ) | ( | ) | ||||
| Cash acquired in business combination | ||||||||
| Acquisitions of intangible assets | ( | ) | ||||||
| Net cash used in investing activities | ( | ) | ( | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Cash proceeds from issuance of common stock, net of fees and costs | ||||||||
| Cash proceeds from sale of units in IPO, net of fees and costs | ||||||||
| Sale of common stock and warrants, net of fees and costs | ||||||||
| Cash proceeds received from exercise of warrants | ||||||||
| Payment of offering costs | ( | ) | ||||||
| Repurchase of shares in litigation settlement | ( | ) | ||||||
| Net cash (used in) provided by financing activities | ( | ) | ||||||
| Effect of foreign currency translation on cash | ( | ) | ||||||
| NET CHANGE IN CASH | ( | ) | ||||||
| Cash - Beginning of period | ||||||||
| Cash - End of period | $ | $ | ||||||
| SUPPLEMENTAL CASH FLOW INFORMATION: | ||||||||
| Equity purchase consideration for acquired business | ||||||||
| Equity purchase consideration for acquired intangible assets | ||||||||
| Lease liabilities arising from obtaining right-of-use assets | ||||||||
| Initial recording of warrant liabilities | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
PASITHEA THERAPEUTICS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2022 AND 2021
NOTE 1 – NATURE OF THE ORGANIZATION AND BUSINESS
Pasithea Therapeutics Corp. (“Pasithea” or the “Company”) was incorporated in the State of Delaware on May 12, 2020 and completed an Initial Public Offering (the “Initial Public Offering”) on September 17, 2021. The Company is a biotechnology company focused on the discovery, research and development of innovative treatments for central nervous system (CNS) disorders and other diseases. The Company is leveraging its expertise in the fields of neuroscience, translational medicine, and drug development to advance new molecular entities that target the pathophysiology underlying such diseases with the goal of bringing life-changing therapies to patients.
The Company’s therapeutic pipeline currently consists of four programs. The Company’s lead product candidate, PAS-004, is a next-generation macrocyclic mitogen-activated protein kinase, or MEK inhibitor that the Company believes may address the limitations and liabilities associated with existing drugs targeting a similar mechanism of action. The remaining three programs are in the discovery stage, which the Company believes address limitations in the treatment paradigm of the indications the Company plans to address with these programs, which are currently amyotrophic lateral sclerosis (“ALS”), multiple sclerosis (“MS”) and schizophrenia.
The Company’s Clinics segment was focused on providing business support services to anti-depression clinics in the U.K. and in the United States. Its operations in the U.K. involved providing business support services to registered healthcare providers who assess patients and, if appropriate, administer intravenous infusions of ketamine. Its operations in the United States involved providing business support services to entities that furnish similar services to patients who personally pay for those services. Operations in the U.K. and the United States were conducted through partnerships with healthcare providers and the Company did not provide professional medical services or psychiatric assessments.
Prior to the date hereof of this Annual Report on Form 10-K, we have discontinued our at-home services in New York, NY as well as our services in the U.K. In addition, we have discontinued our clinical operations in Los Angeles, CA and are actively exploring options for the disposal of related property. Accordingly, as of the date of this Annual Report on Form 10-K, we have discontinued the operations of our Clinics segment.
Throughout this report, the terms “our,” “we,” “us,” and the “Company” refer to Pasithea Therapeutics Corp. and its subsidiaries, Pasithea Therapeutics Limited (U.K.), Pasithea Therapeutics Portugal, Sociedade Unipessoal Lda, Pasithea Clinics Corp, Alpha-5 Integrin, LLC (See Note 6), and AlloMek Therapeutics, LLC (See Note 6). Pasithea Therapeutics Limited (U.K.) is a private limited Company, registered in the United Kingdom (U.K.). Pasithea Therapeutics Portugal, Sociedade Unipessoal Lda is a private limited Company, registered in Portugal. Pasithea Clinics Corp. is incorporated in Delaware. Alpha-5 Integrin, LLC is Delaware limited liability company. AlloMek Therapeutics, LLC is Delaware limited liability company.
Basis of Presentation
The accompanying audited consolidated financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
Emerging Growth Company
The Company is an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and it may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and approval of any golden parachute payments not previously approved. Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s consolidated financial statements with another public company which is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
F-7
Liquidity and Capital Resources
As
of December 31, 2022, the Company had approximately $
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES AND NEW ACCOUNTING STANDARDS
Principles of Consolidation
The Company evaluates the need to consolidate affiliates based on standards set forth in Accounting Standards Codification (“ASC”) 810, “Consolidation,” (“ASC 810”). The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries, Pasithea Therapeutics Limited (U.K.), Pasithea Clinics Corp. (“Pasithea Clinics”) Pasithea Therapeutics Portugal, Sociedade Unipessoal Lda (“Pasithea Portugal”), Alpha-5 Integrin, LLC, and AlloMek Therapeutics, LLC. All significant intercompany transactions and balances have been eliminated in consolidation.
These consolidated financial statements are presented in U.S. Dollars.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires the Company’s management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statement and the reported amounts of revenues and expenses during the reporting period.
Making estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the financial statements, which management considered in formulating its estimate, could change in the near term due to one or more future confirming events. Management regularly makes estimates related to the fair value of warrant liabilities; the recoverability of long-lived assets; the fair values and useful lives of intangible assets acquired in business combinations; the potential impairment of goodwill; and income taxes. The Company bases its estimates on historical experience and on various assumptions that are believed to be reasonable, the results of which form the basis for the amounts recorded in the consolidated financial statements. As appropriate, the Company obtains reports from third-party valuation experts to inform and support estimates related to fair value measurements.
Research and Development
Research and development costs are charged to operations when incurred and are included in operating expense, except for goodwill related to intellectual property & patents. Research and development costs consist principally of compensation of employees and consultants that perform the Company’s research activities, payments to third parties for preclinical and non-clinical activities, costs to acquire drug product from contract development and manufacturing organizations and third-party contractors relating to chemistry, manufacturing and controls (“CMC”) efforts, the fees paid for and to maintain the Company’s intellectual property, and research and development costs related to our discovery programs. Depending upon the timing of payments to the service providers, the Company recognizes prepaid expenses or accrued expenses related to these costs. These accrued or prepaid expenses are based on management’s estimates of the work performed under service agreements, milestones achieved and experience with similar contracts. The Company monitors each of these factors and adjusts estimates accordingly.
F-8
Research and development also includes contra expense related to costs reimbursed under the Company’s grant agreement.
Grants
In connection with the acquisition
of Alpha-5 integrin (“Alpha-5”), the Company legally assumed rights under a grant agreement with FightMND, which was entered
into by Alpha-5 on September 23, 2021. FightMND supports pre-clinical research, development and assessment of therapeutics for Motor Neuron
Disease, including ALS. Under the grant agreement, the Company is entitled to reimbursements for costs incurred for research related to
monoclonal antibody targeting a5b1
integrin as a potential treatment for ALS. For the year ended December 31, 2022, the Company recorded $
Cash and Cash Equivalents
The Company considers all short-term investments with an original maturity of three months or less when purchased to be cash equivalents. The Company had no cash equivalents as of December 31, 2022 and December 31, 2021.
Property and Equipment
Property and equipment is recorded at cost. Depreciation is computed using straight-line and accelerated methods over the estimated useful lives of the related assets. Expenditures that enhance the useful lives of the assets are capitalized and depreciated. Maintenance and repairs are expensed as incurred. When properties are retired or otherwise disposed of, related costs and related accumulated depreciation are removed from the accounts.
Offering Costs
Offering
costs consist of professional fees, filing, regulatory and other costs incurred through the balance sheet date that are directly related
to the Initial Public Offering. In September 2021, the Company recognized offering costs of $
Warrant Liability
The Company accounts for its Public and Representative Warrants (each, the “Public Warrants” and “Representative Warrants” and, collectively, the “IPO Warrants”) in accordance with the guidance contained in ASC 815, “Derivatives and Hedging,” under which the IPO Warrants do not meet the criteria for equity treatment and must be recorded as derivative liabilities. Accordingly, the Company classifies the IPO Warrants as liabilities at their fair value and adjusts the IPO Warrants to fair value at each reporting period. This liability is subject to re-measurement at each balance sheet date until the IPO Warrants are exercised or expire, and any change in fair value is recognized in the Company’s consolidated statement of operations and comprehensive loss. The fair value of the Public and Representative Warrants was initially measured at the end of each reporting period, using a Black-Scholes option pricing model. As of December 31, 2022, the fair value of the Public Warrants was measured using quoted market prices, and the fair value of the Representative Warrants was based on an estimate of the relative fair value to the Public Warrants, accounting for a small difference in the exercise price.
Income Taxes
The
Company follows the asset and liability method of accounting for income taxes under ASC 740, “Income Taxes.” Deferred tax
assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement
carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured
using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered
or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that included
the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be
realized. As of December 31, 2022, the Company had deferred tax assets related to certain net operating losses. A valuation allowance
was established against these deferred tax assets at their full amount, resulting in a
F-9
ASC 740 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities. The Company recognizes accrued interest and penalties related to unrecognized tax benefits as income tax expense. There were no unrecognized tax benefits and no amounts accrued for interest and penalties as of December 31, 2022 and December 31, 2021. The Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation from its position. The Company is subject to income tax examinations by major taxing authorities since inception.
Concentration of Credit Risk
Financial
instruments that potentially subject the Company to concentrations of credit risk consist of a cash account in a financial institution,
which, at times, may exceed the Federal Depository Insurance Coverage of $
Fair Value of Financial Instruments
With the exception of liabilities related to the IPO Warrants, described in the table below, the fair value of the Company’s assets and liabilities, which qualify as financial instruments under ASC 820, “Fair Value Measurements and Disclosures,” approximates the carrying amounts represented in the accompanying balance sheet, primarily due to their short-term nature.
Fair Value Measurements
Fair value is defined as the price that would be received for sale of an asset or paid for transfer of a liability, in an orderly transaction between market participants at the measurement date. GAAP establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include:
| ● | Level 1, defined as observable inputs such as quoted prices (unadjusted) for identical instruments in active markets; |
| ● | Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and |
| ● | Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable. |
F-10
The following table presents information about the Company’s liabilities that are measured at fair value on a recurring basis and indicates the fair value hierarchy of the valuation inputs the Company utilized to determine such fair value:
| Fair value measurements at reporting date using: | ||||||||||||||||
| Description | Fair Value | Quoted
prices (Level 1) | Significant (Level 2) | Significant (Level 3) | ||||||||||||
| Liabilities: | ||||||||||||||||
| Public Warrant liabilities, December 31, 2022 | $ | $ | $ | $ | ||||||||||||
| Representative Warrant liabilities, December 31, 2022 | $ | $ | $ | $ | ||||||||||||
| Liabilities: | ||||||||||||||||
| Public Warrant liabilities, December 31, 2021 | $ | $ | $ | $ | ||||||||||||
| Representative Warrant liabilities, December 31, 2021 | $ | $ | $ | $ | ||||||||||||
The following table presents a reconciliation of the Level 3 representative warrant liabilities:
| Representative warrant liabilities, September 17, 2021 | ||||
| Issuances | ||||
| Exercises | ||||
| Change in fair value | ( | ) | ||
| Representative warrant liabilities, December 13, 2021 | ||||
| Issuances | ||||
| Exercises | ||||
| Changes in fair value | ( | ) | ||
| Representative warrant liabilities, December 31, 2022 |
The change in fair value of the representative warrant liabilities is recorded in Change in fair value of warrant liabilities on the Consolidated Statement of Operations and Comprehensive Loss.
The fair value of the liability associated with the Public Warrants as of December 31, 2022 was based on the quoted closing price on The Nasdaq Capital Market and is classified as Level 1. The fair value of the liability associated with the Representative Warrants as of December 31, 2022 was based on an estimate of the relative fair value to the Public Warrants, accounting for a small difference in the exercise price, and is classified as Level 3. The change of the Public Warrant liability from Level 3 to Level 1 was the only change between levels of the fair value hierarchy from December 31, 2021 to December 31, 2022.
In some circumstances, the inputs used to measure fair value might be categorized within different levels of the fair value hierarchy. In those instances, the fair value measurement is categorized in its entirety in the fair value hierarchy based on the lowest level input that is significant to the fair value measurement.
Revenue
The Company accounts for revenue in accordance with ASC Topic 606, “Revenue from Contracts with Customers.”
The
Company currently derives all its revenue from its operations providing business support services to registered healthcare providers
who assess patients, and if appropriate, administer intravenous infusions of ketamine. Under the business support services agreements,
the Company, among other things, markets the treatments to the extent permitted under law, arranges and pays for the fit-out of the consulting
room, provides equipment necessary for the treatments, develops, operates and maintains a booking website for the treatments, makes bookings
and takes payments, and employs or engages customer service advisers to liaise with clinical staff and pay certain staff costs. The price
of the treatments are fixed amounts jointly established by the Company and the healthcare providers. The Company collects
The Company also may arrange psychotherapy sessions with independent therapy professionals for patients. In such cases, the Company acts as a principal and recognizes the gross amount of revenue earned from such sessions, with the cost paid to the independent therapy professionals recognized in cost of services in the consolidated statements of operations and comprehensive loss.
The Company’s performance obligation is satisfied when the services are rendered to the customer. There were no contract assets or liabilities as of December 31, 2022 or December 31, 2021. All sales have fixed pricing and there are currently no variable components included in the Company’s revenue.
F-11
Net Loss Per Share
Net
loss per share is computed by dividing net loss by the weighted average number of shares of common stock par value $
Foreign Currency Translations
The Company’s functional and reporting currency is the U.S. dollar. All transactions initiated in other currencies are translated into U.S. dollars using the exchange rate prevailing on the date of transaction. Monetary assets and liabilities denominated in foreign currencies are translated into the U.S. dollar at the rate of exchange in effect at the balance sheet date. Unrealized exchange gains and losses arising from such transactions are deferred until realization and are included as a separate component of stockholders’ equity (deficit) as a component of comprehensive income or loss. Upon realization, the amount deferred is recognized in income in the period when it is realized.
Translation of Foreign Operations
The financial results and position of foreign operations whose functional currency is different from the Company’s presentation currency are translated as follows:
| ● | assets and liabilities are translated at period-end exchange rates prevailing at that reporting date; |
| ● | equity is translated at historical exchange rates; and |
| ● | income and expenses are translated at average exchange rates for the period. |
Exchange differences arising on translation of foreign operations are transferred directly to the Company’s accumulated other comprehensive loss in the consolidated financial statements. Transaction gains and losses arising from exchange rate fluctuation on transactions denominated in a currency other than the functional currency are included in the consolidated statements of operations and comprehensive loss.
The relevant translation rates are as follows:
| December 31, 2022 | December 31, 2021 | |||||||
| Closing rate, British Pound (GBP) to US$ at period end | ||||||||
| Average rate, GBP to US$ for the period ended | ||||||||
| Closing rate, Euro (EUR) to US$ at period end | ||||||||
| Average rate, EUR to US$ for the period ended | ||||||||
Comprehensive Loss
ASC 220, “Comprehensive Income,” establishes standards for reporting and display of comprehensive loss and its components in a full set of general-purpose financial statements. As of December 31, 2022 and December 31, 2021, the Company had no items impacting other comprehensive income (loss) except for the foreign currency translation adjustment.
F-12
Acquisitions, Intangible Assets and Goodwill
The consolidated financial statements reflect the operations of an acquired business beginning as of the date of acquisition. Assets acquired and liabilities assumed are recorded at their fair values at the date of acquisition; goodwill is recorded for any excess of the purchase price over the fair values of the net assets acquired. Significant judgment is required to determine the fair value of certain tangible and intangible assets and in assigning their respective useful lives. Accordingly, we typically obtain the assistance of third-party valuation specialists for significant tangible and intangible assets. The fair values are based on available historical information and on future expectations and assumptions deemed reasonable by management but are inherently uncertain. The Company typically employs an income method to measure the fair value of intangible assets, which is based on forecasts of the expected future cash flows attributable to the respective assets. Significant estimates and assumptions inherent in the valuations reflect a consideration of other marketplace participants and include the amount and timing of future cash flows (including expected growth rates and profitability), the underlying product or technology life cycles, economic barriers to entry and the discount rate applied to the cash flows. Unanticipated market or macroeconomic events and circumstances could affect the accuracy or validity of the estimates and assumptions. Determining the useful life of an intangible asset also requires judgment. Intangible assets are amortized over their estimated lives. Any intangible assets associated with acquired in-process research and development activities (“IPR&D”) are not amortized until a product is available for sale.
Impairment of Long-Lived Assets and Goodwill
Long-lived and amortizable intangible assets are assessed annually for impairment or sooner should impairment indicators exist. Significant events or changes in business circumstances indicate that the carrying value of the assets may not be recoverable. Such circumstances may include a significant decrease in the market price of an asset, a significant adverse change in the manner in which the asset is being used or in its physical condition or a history of operating or cash flow losses associated with the use of an asset. An impairment loss is recognized when the carrying amount of an asset exceeds the anticipated future undiscounted cash flows expected to result from the use of the asset and its eventual disposition. The amount of the impairment loss is the excess of the asset’s carrying value over its fair value. There were no charges related to impairments of long-lived assets for all periods presented.
Goodwill represents the excess of the purchase price over the fair value of the identifiable net assets acquired in a business combination. Goodwill is assessed for impairment annually during the fourth quarter, or more frequently if impairment indicators exist. Impairment exists when the carrying amount of goodwill exceeds its implied fair value. The Company may elect to assess goodwill for impairment using a qualitative or a quantitative approach, to determine whether it is more likely than not that the fair value of goodwill is greater than its carrying value. There were no charges related to goodwill impairment for all periods presented.
Leases
The Company’s has leases related to office space. The Company determines whether a contract is or contains a lease at the time of the contract’s inception based on the presence of identified assets and the Company’s right to obtain substantially all the economic benefit from or to direct the use of such assets. When the Company determines a lease exists, it records a right-of-use (“ROU”) asset and corresponding lease liability on its balance sheet. ROU assets represent the Company’s right to use an underlying asset for the lease term. Lease liabilities represent the Company’s obligation to make lease payments arising from the lease. ROU assets are recognized at the lease commencement date at the present value of the remaining future lease payments the Company is obligated for under the terms of the lease. Lease liabilities are recognized concurrent with the recognition of the ROU asset and represent the present value of lease payments to be made under the lease. These ROU assets and liabilities are adjusted for any prepayments, lease incentives received, and initial direct costs incurred. As the discount rate implicit in the lease is not readily determinable in most of the Company’s leases, the Company uses its incremental borrowing rate based on the information available at the lease commencement date in determining the present value of lease payments. If the Company’s lease terms include an option to extend the lease for a set period, the Company evaluates the renewal option and should it be reasonably certain that the Company will exercise that option, adjusts the ROU asset and liability accordingly.
Stock-Based Compensation
The Company accounts for its stock-based compensation awards to employees and members of its Board (the “Board”) in accordance with ASC Topic 718, Compensation—Stock Compensation (“ASC 718”). ASC 718 requires all stock-based payments to employees and Board members, including grants of employee stock options, to be recognized in the statements of operations by measuring the fair value of the award on the date of grant and recognizing this fair value as stock-based compensation using a straight-line method over the requisite service period, generally the vesting period.
F-13
The Company estimates the grant date fair value of stock option awards using the Black-Scholes option-pricing model. The use of the Black-Scholes option-pricing model requires management to make assumptions with respect to the expected term of the option, the expected volatility of the Common Stock consistent with the expected life of the option, risk-free interest rates and expected dividend yields of the Common Stock.
Recent Accounting Pronouncements
In June 2022, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2022-03, Fair Value Measurement (Topic 820) (“ASU 2022-03”). The amendments in ASU 2022-03 clarify that a contractual restriction on the sale of an equity security is not considered part of the unit of account of the equity security and, therefore, is not considered in measuring fair value. The amendments also clarify that an entity cannot, as a separate unit of account, recognize and measure a contractual sale restriction. The amendments in this Update also require additional disclosures for equity securities subject to contractual sale restrictions. The provisions in this Update are effective for fiscal years beginning after December 15, 2024. Early adoption is permitted. The Company does not expect to early adopt this ASU. The Company is currently evaluating the impact of adopting this guidance on the consolidated balance sheets, results of operations and financial condition.
The Company does not believe that any other recently issued, but not yet effective, accounting pronouncements, if currently adopted, would have a material effect on the Company’s financial statements.
NOTE 3 – INITIAL PUBLIC OFFERING
Pursuant
to the Initial Public Offering, on September 17, 2021, the Company sold
Each Unit consisted of one share of Common Stock and one Public Warrant. The Common Stock and Public Warrants are listed on The Nasdaq Capital Market under the symbols “KTTA” and “KTTAW,” respectively. Each redeemable Public Warrant entitles the holder to immediately purchase one share of Common Stock at an exercise price of $6.25 per share, and expire five years from issuance.
In connection with the Initial Public Offering,
the Company granted the underwriters an option for a period of 45 days to purchase up to an additional
The Company classifies each Public Warrant as a liability at its fair value and the Public Warrants were allocated a portion of the proceeds from the issuance of the Units equal to its fair value determined by the Black-Scholes model.
NOTE 4 – PROPERTY AND EQUIPMENT
Property and equipment, net consists of the following (in thousands):
| As of December 31, | ||||||||
| 2022 | 2021 | |||||||
| Leasehold improvements | $ | $ | ||||||
| Medical equipment | ||||||||
| Office equipment | ||||||||
| Property and equipment, gross | ||||||||
| Less: accumulated depreciation | ( | ) | ( | ) | ||||
| Property and equipment, net | $ | $ | ||||||
F-14
Depreciation
expense was $
NOTE 5 – LEASES
Medical Office Lease – West Hollywood, California
In
March 2022, the Company entered into an agreement to lease a medical office in West Hollywood, California. The lease commenced on April
1, 2022.
This
lease was accounted for under ASC 842, Leases, which resulted in the recognition of a right of use asset (“ROU asset”) and
liability of $
Laboratory Lease – South San Francisco, California
In
August 2022, the Company, as a lessee, entered into an amended sublease agreement to sublease laboratory and office space in South San
Francisco, California. The lease commenced on August 15, 2022. The term of this sublease is for a period of thirty-nine and one-fourth
(39.25) months commencing on the effective date, until May 15,2024. The lease has a gross monthly rent of $
This
lease was accounted for as an operating lease under ASC 842, Leases, which resulted in the recognition of a right of use asset (“ROU
asset”) and liability of approximately $
As of December 31, 2022, the Company recognized total ROU assets and lease liabilities of as follows:
| Non-current leases – right of use assets | $ | |||
| Current liabilities – operating lease liabilities | $ | |||
| Non-current liabilities – operating lease liabilities | $ |
The following table provides a summary of other information related to leases for the year ended December 31, 2022:
| Operating lease expense | $ | |||
| Cash paid for amounts included in the measurement of operating lease liabilities | $ |
F-15
The following table summarizes the maturity of the Company’s operating lease payments as of December 31, 2022:
| Period | Amount | |||
| 2023 | $ | |||
| 2024 | $ | |||
| 2025 | $ | |||
| 2026 | $ | |||
| 2027 | $ | |||
NOTE 6 – ACQUISITIONS
Business Combination with Alpha-5 Integrin LLC
On
June 21, 2022, the Company entered into a membership purchase agreement (the “Alpha-5 Agreement”) with Alpha-5 to purchase
In addition, the Alpha-5 Agreement allows for an earnout to be paid as part of the consideration due to the sellers. As any future sales are predicated upon FDA approval, no amounts will be due the sellers in the absence of that approval. Should FDA approval be obtained the amount of the earnout payment is dependent on the attainment of certain financial targets. The terms of the earnout contain three performance target thresholds that trigger three different payout amounts depending on which of the three targets is achieved. Sales generated after the drug is no longer subject to any patent protection or regulatory exclusivity are excluded from the earnout calculation. Purchase consideration consisted of the following:
| Equity consideration | $ | |||
| Warrants issued as consideration | ||||
| Total purchase consideration | $ |
The Alpha-5 acquisition was accounted for as a business combination in accordance with ASC 805, Business Combinations. The fair values of the acquired assets and liabilities as of the acquisition date were:
| Cash | $ | |||
| Prepaid assets | ||||
| Fixed assets | ||||
| In-process research and development | ||||
| Accounts payable & accrued expenses | ( | |||
| Net assets acquired, excluding Goodwill | ||||
| Goodwill |
The
goodwill recognized is largely attributable to the potential leveraging of Alpha-5’s scientific expertise in the integrin space.
The Company believes the acquisition of Alpha-5 will help in its efforts to move the treatment forward and increase its potential to
have a positive impact on the treatment of ALS. This goodwill is expected to be deductible for income tax purposes. Expenses incurred
in relation to this acquisition totaled approximately $
F-16
Asset Acquisition of AlloMek Therapeutics, LLC
On
October 11, 2022, the Company entered into a membership interest purchase agreement, whereby the Company acquired
The AlloMek acquisition was accounted for as an asset acquisition as substantially all of the fair value of the gross assets acquired is attributable to CIP-137401. The cost of the asset acquisition included the purchase consideration as well as transaction expenses, as follows:
| $ | ||||
| Warrants to acquire | ||||
| Cash | ||||
| Non-refundable payment for AlloMek transaction expenses | ||||
| Pasithea transaction expenses | ||||
| Total cost of asset acquisition | $ |
The Company capitalized the cost of the asset acquisition as intellectual property within Intangibles on the consolidated balance sheet as of December 31, 2022.
NOTE 7 – INTANGIBLE ASSETS AND GOODWILL
Intangible assets, net consists of the following (in thousands):
| December 31, 2022 | ||||||||||||
| Gross Carrying Amount | Accumulated Amortization | Net | ||||||||||
| In-process research and development | ||||||||||||
| Patents and intellectual property | ( | ) | ||||||||||
| Intangible assets, net | ( | ) | ||||||||||
As of December 31, 2022, future expected amortization expense of Intangible assets was as follows:
| 2023 | ||||
| 2024 | ||||
| 2025 | ||||
| 2026 | ||||
| 2027 |
During
the year ended December 31, 2022, the Company acquired $
F-17
NOTE 8 – STOCKHOLDERS’ EQUITY
The
Company is authorized to issue an aggregate of
Effective April 8, 2021, the Company amended its certificate of incorporation to effect a one-for-twenty (1:20) reverse stock split of our outstanding shares of Common Stock. No fractional shares were issued as a result of the reverse stock split. Any fractional shares resulting from the reverse stock split were paid in cash. The reverse stock split did not otherwise affect any of the rights currently accruing to holders of our Common Stock. All share information presented in these financial statements has been retroactively adjusted to reflect the reduced number of shares of Common Stock outstanding.
Common Stock
The
Company had
Each
holder of Common Stock is entitled to
In addition, the holders of our Common Stock will be entitled to receive ratably such dividends, if any, as may be declared by the Board out of legally available funds; however, the current policy of our Board is to retain earnings, if any, for operations and growth. Upon liquidation, dissolution or winding-up, the holders of our Common Stock will be entitled to share ratably in all assets that are legally available for distribution.
Holders of our Common Stock have no preemptive, conversion or subscription rights, and there are no redemption or sinking fund provisions applicable to the Common Stock. The rights, preferences and privileges of the holders of Common Stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of our preferred stock that we may designate and issue in the future.
2021 Stock Incentive Plan
The
Company’s Board (the “Board”) and stockholders have adopted and approved the 2021 Stock Incentive Plan (the “2021
Plan”) which took effect on July 15, 2021. The 2021 Plan allows for the issuance of securities, including stock options, restricted
stock, and restricted stock units (“RSUs”) to employees, Board members and consultants. The initial number of shares
of Common Stock available for issuance under the 2021 Plan was
As
of January 1, 2022, the number of shares of Common Stock available for issuance under the 2021 Plan automatically increased by
2021 Common Stock Transactions
Prior
to the Initial Public Offering, during 2021, the Company entered into various subscription agreements in connection with a private placement
of Common Stock at a price of $
F-18
Prior
to the Initial Public Offering, during 2021, the Company entered into various subscription agreements in connection with a second private
placement of Common Stock at a price of $
Prior
to the Initial Public Offering, during 2021, the Company issued an additional
During
the year ended December 31, 2021, the Company issued an aggregate of
During
the year ended December 31, 2021, the Company issued an aggregate of
November 2021 Private Placement
On
November 24, 2021, the Company entered into a securities purchase agreement (the “November 2021 Purchase Agreement”) pursuant
to which the Company agreed to sell, in a private placement (the “November 2021 Private Placement”) with institutional investors
The
investors may exercise the PIPE Warrants on a cashless basis if the shares of Common Stock underlying the PIPE Warrants are not then
registered pursuant to an effective registration statement. The investors have contractually agreed to restrict their ability to exercise
the PIPE Warrants such that the number of shares of Common Stock held by the investors and any of their affiliates after such exercise
does not exceed either
In connection with the November 2021 Purchase Agreement, the Company entered into a registration rights agreement (the “November 2021 Registration Rights Agreement”) with the investors. Pursuant to the November 2021 Registration Rights Agreement, the Company was required to file a resale registration statement with the Securities and Exchange Commission (the “SEC”) to register for resale the shares and the warrant shares and to have such registration statement declared effective within 60 days after the date of the Purchase Agreement, or 90 days of the date of the November 2021 Purchase Agreement in the event the registration statement is subject to a “full review” by the SEC. The Company is obligated to pay certain cash liquidated damages to the investor if it fails to file the resale registration statement when required, fail to cause the registration statement to be declared effective by the SEC when required, or if it fails to maintain the effectiveness of the registration statement. The registration statement was declared effective by the SEC on December 16, 2021.
Pursuant
to a placement agent agreement (the “Placement Agent Agreement”), dated as of November 24, 2021 the Company paid the placement
agent a cash fee of
On
November 29, 2021, the Company consummated the November 2021 Private Placement, pursuant to which it issued an aggregate of
2022 Common Stock Transactions
During
the year ended December 31, 2022, the Company issued
F-19
During the year ended
December 31, 2022, the Company issued
During the year ended
December 31, 2022, the Company entered into a comprehensive Settlement and Cooperation Agreement (“the Settlement and Cooperation
Agreement”) with certain shareholders (collectively, the “Camac Group”) resolving all outstanding issues between the
parties. Pursuant to the Settlement and Cooperation Agreement, the Camac Group agreed to a three-year standstill provision and the parties
agreed to dismiss with prejudice the pending Delaware litigation against the Company and the Board filed by the Camac Group. The Company
purchased all
All repurchased shares were cancelled and recorded within Accumulated Deficit on the Company’s consolidated balance sheet as of December 31, 2022. The Company recorded the reimbursed fees and expenses within Litigation settlements on the consolidated statement of operations for the year ended December 31, 2022.
During the year ended December 31, 2022, the Company
entered into settlement agreements with certain shareholders for approximately $
Restricted Stock Units
During the year ended December 31, 2021, the Company issued its chief
executive officer, Dr. Marques,
Restricted Stock
During
the year ended December 31, 2022, the Company issued an aggregate of
During
the year ended December 31, 2021, the Company issued
During
the year ended December 31, 2021, the Company recognized approximately $
NOTE 9 – STOCK OPTIONS
During
the year ended December 31, 2022, the Company issued stock options under the 2021 Plan to employees, to purchase an aggregate of
During
the year ended December 31, 2022, the Company issued stock options under the 2021 Plan to its current chief financial officer, to purchase
an aggregate of
F-20
During
the year ended December 31, 2022, the Company issued stock options under the 2021 Plan to a non-executive Board member, to purchase an
aggregate of
During
the year ended December 31, 2022, stock options to purchase an aggregate of
During
the year ended December 31, 2022, stock options to purchase an aggregate of
During
the year ended December 31, 2022, the Company recorded approximately $
During
the year ended December 31, 2021, the Company issued stock options under the 2021 Plan to three non-executive Board members, to purchase
an aggregate of
During
the year ended December 31, 2021, the Company recorded approximately $
Stock option activity for the years ended December 31, 2022 and 2021 was as follows:
Number of Options | Weighted
average exercise price per share | Weighted-average remaining contractual term (years) | Aggregate intrinsic value (in thousands) | |||||||||||||
| Outstanding, January 1, 2021 | $ | $ | ||||||||||||||
| Granted | $ | $ | ||||||||||||||
| Expired/Cancelled | $ | $ | ||||||||||||||
| Exercised | $ | $ | ||||||||||||||
| Outstanding, December 31, 2021 | $ | $ | ||||||||||||||
| Exercisable, December 31, 2021 | $ | $ | ||||||||||||||
| Outstanding, January 1, 2022 | $ | $ | ||||||||||||||
| Granted | $ | $ | ||||||||||||||
| Expired/Cancelled | ( | ) | $ | $ | ||||||||||||
| Exercised | $ | $ | ||||||||||||||
| Outstanding, December 31, 2022 | $ | $ | ||||||||||||||
| Exercisable, December 31, 2022 | $ | $ | ||||||||||||||
As
of December 31, 2022, remaining unamortized stock-based compensation expense related to the stock options was $
F-21
The Company estimates the fair value of each stock option on the date of grant using the Black-Scholes option pricing model, which requires various assumptions including fair value of the underlying share, volatility, expected option life, risk-free interest rate and expected dividends. The fair value of the underlying share was based on the fair value on the grant date. The expected term was based on the expected exercise behavior of grantees. Expected volatility was calculated based on the volatilities of a peer group of companies. The risk-free rate of the option is based on the U.S. Treasury rate for the expected term of the option. The following weighted-average assumptions were used in the Black-Scholes calculations:
| Year ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Expected volatility | % | % | ||||||
| Expected term (in years) | ||||||||
| Weighted-average risk-free interest rate | % | % | ||||||
| Weighted average fair value of underlying interest | $ | $ | ||||||
| Expected dividends | ||||||||
The
weighted average grant date fair value of options granted during the years ended December 31, 2022 and 2021 was $
NOTE 10 – WARRANTS
AlloMek Warrants
During
the year ended December 31, 2022, the Company issued warrants to purchase an aggregate of
As
of December 31, 2022,
Alpha-5 Warrants
During
the year ended December 31, 2022, the Company issued warrants to purchase an aggregate of
As
of December 31, 2022,
F-22
PIPE Warrants
During
the year ended December 31, 2021, the Company issued PIPE Warrants to purchase an aggregate of
As
of December 31, 2022 and 2021,
IPO Warrants
During
the year ended December 31, 2021, the Company issued Public Warrants to purchase an aggregate of
Simultaneously
with the consummation of the closing of the Initial Public Offering, the Company issued the underwriters a total of
The Company evaluated the IPO Warrants as either equity-classified or liability-classified instruments based on an assessment of the IPO Warrants’ specific terms and applicable authoritative guidance in ASC 480, “Distinguishing Liabilities from Equity” (“ASC 480”) and ASC 815, “Derivatives and Hedging” (“ASC 815”). The assessment considers whether the IPO Warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the IPO Warrants meet all of the requirements for equity classification under ASC 815, including whether the IPO Warrants are indexed to the Company’s own common stock, among other conditions for equity classification. Pursuant to such evaluation, the Company further evaluated the IPO Warrants under ASC 815-40, Derivatives and Hedging — Contracts in Entity’s Own Equity, and concluded that the IPO Warrants do not meet the criteria to be classified in stockholders’ equity.
As
of December 31, 2022
As
of December 31, 2022, the fair value of the Public Warrants was approximately $
During
the year ended December 31, 2021,
As
of December 31, 2021
As of December 31, 2021, the fair value of the
Public Warrants was approximately $
F-23
Warrant activity for the years ended December 31, 2022 and 2021 was as follows:
Number of Warrants | Exercise price per share | Weighted average exercise price | ||||||||
| Outstanding and exercisable on January 1, 2021 | - | |||||||||
| Granted | $ | $ | ||||||||
| Expired/Cancelled | - | |||||||||
| Exercised | ( | ) | $ | $ | ||||||
| Outstanding and exercisable on December 31, 2021 | $ | $ | ||||||||
| Outstanding and exercisable on January 1, 2022 | $ | $ | ||||||||
| Granted | $ | $ | ||||||||
| Expired/Cancelled | - | |||||||||
| Exercised | - | |||||||||
| Outstanding and exercisable on December 31, 2022 | $ | $ | ||||||||
Warrants exercisable at December 31, 2022 were as follows:
| Exercise Price | Number of Warrants | Weighted-average
remaining contractual term (years) | Weighted-average
exercise price | |||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
NOTE 11 – INCOME TAXES
The Company accounts for income taxes under ASC 740 - Income Taxes (“ASC 740”), which provides for an asset and liability approach of accounting for income taxes. Under this approach, deferred tax assets and liabilities are recognized based on anticipated future tax consequences, using currently enacted tax laws, attributed to temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts calculated for income tax purposes.
Significant components of the Company’s deferred tax assets as of December 31, 2022 and 2021 are summarized below.
| December 31, 2022 | December 31, 2021 | |||||||
| Deferred tax assets: | ||||||||
| Amortization | $ | $ | ||||||
| Research & development costs | ||||||||
| ROU asset | ||||||||
| Warrant liabilities | ||||||||
| Stock-based compensation | - | |||||||
| Net operation loss carryforwards | ||||||||
| Total deferred tax asset | ||||||||
| Deferred tax liabilities: | ||||||||
| Depreciation | ( | ) | ( | ) | ||||
| Net deferred tax asset | ||||||||
| Valuation allowance | ( | ) | ( | ) | ||||
| $ | $ | |||||||
F-24
The Company recognizes deferred tax assets to
the extent that it believes that these assets are more likely than not to be realized. In making such a determination, the Company considers
all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable
income, tax-planning strategies, and results of recent operations. The Company assessed the need for a valuation allowance against its
net deferred tax assets and determined a full valuation allowance is required since the Company has no history of generating taxable income.
Our deferred tax asset and valuation allowance increased by $
A reconciliation of the federal income tax rate to the Company’s effective tax rate at December 31, 2022 and 2021 is as follows:
| December 31, 2022 | December 31, 2021 | |||||||
| Statutory federal income tax rate | % | % | ||||||
| State taxes, net of federal tax benefit | % | % | ||||||
| Stock-based compensation | % | ( | )% | |||||
| Shares issued for services | % | ( | )% | |||||
| Change in fair value of warrant liabilities | % | % | ||||||
| Permanent items | ( | )% | - | % | ||||
| Other | % | % | ||||||
| Change in valuation allowance | ( | )% | ( | )% | ||||
| Income tax provision | % | % | ||||||
The Company’s ability to utilize net operating
loss carryforwards will depend on its ability to generate adequate future taxable income. Future utilization of the net operating loss
carry forwards is subject to certain limitations under Section 382 of the Internal Revenue Code. As of December 31, 2022, the Company
had federal and state net operating loss carryforwards available to offset future taxable income in the amounts of approximately $
The Company has evaluated its income tax positions and has determined that it does not have any uncertain tax positions. The Company will recognize interest and penalties related to any uncertain tax positions through its income tax expense.
The Company is subject to franchise tax filing requirements in the State of Delaware.
NOTE 12 – NET LOSS PER COMMON SHARE
Basic net loss per share is computed by dividing net loss available to Common Stockholders by the weighted average number of common shares outstanding during the period. Diluted earnings per share reflect, in periods in which they have a dilutive effect, the impact of common shares issuable upon exercise of stock options and warrants and conversion of convertible debt that are not deemed to be anti-dilutive. The dilutive effect of the outstanding stock options and warrants is computed using the treasury stock method.
At
December 31, 2022, diluted net loss per share did not include the effect of
At
December 31, 2021, diluted net loss per share did not include the effect of
F-25
NOTE 13 – SEGMENTS
The Company has the following reportable segments:
Therapeutics (“Therapeutics”): The Therapeutics segment performs activities related to discovery, research and development of innovative treatments for central nervous system (CNS) disorders and other diseases.
Support services to anti-depression clinics in the US and UK (“Clinics”): The Clinics segments provided business support services to anti-depression clinics in the U.K. and in the United States. Its operations in the U.K. involved providing business support services to registered healthcare providers who assess patients and, if appropriate, administer intravenous infusions of ketamine. Its operations in the United States involved providing business support services to entities that furnish similar services to patients who personally pay for those services. Operations in the U.K. and the United States were conducted through partnerships with healthcare providers and the Company does not provide professional medical services or psychiatric assessments.
The Company evaluates the performance of its business segments primarily based on revenues and net income. For the years ended December 31, 2022 and 2021, segment operating results were as follows:
| For the years ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Revenues | ||||||||
| Therapeutics | $ | $ | ||||||
| Clinics | ||||||||
| Total revenues | ||||||||
| Net loss | ||||||||
| Drug Research and Development | ( | ) | ( | ) | ||||
| Clinics | ( | ) | ( | ) | ||||
| Total net loss | $ | ( | ) | $ | ( | ) | ||
As of December 31, 2022, total assets attributable to the Therapeutics
and Clinics segments were $
NOTE 14 – RELATED PARTY TRANSACTIONS
Alpha-5 integrin, LLC
On June 21, 2022, we entered into the Alpha-5
Agreement with PD Joint Holdings, LLC Series 2016-A and Lawrence Steinman (collectively, the “Sellers”), pursuant to which
the Sellers sold all of the issued and outstanding equity of Alpha-5 to the Company. Lawrence Steinman, our Executive Chairman and
Co-Founder, was a
In addition, the Alpha-5 Agreement allows for an earnout payment to be paid as part of the consideration due to the Sellers (the “Earnout Amount”). The Earnout Amount is dependent upon FDA approval of a monoclonal antibody targeting alpha5 beta1 integrin that was in development by Alpha-5 at the time of the execution of the Alpha-5 Agreement. Should such FDA approval be obtained, the Earnout Amount will depend on the attainment of certain net sales targets. The terms of the earnout contain three net sales target thresholds that trigger three different Earnout Amounts depending on which of the three net sales targets is achieved. Net sales generated after the drug is no longer subject to any patent protection or regulatory exclusivity are excluded from the Earnout Amount calculation. The earnout is deemed part of the consideration paid for the acquisition, in the form of contingent consideration. As of December 31, 2022, this amount has been determined by the Company to hold no value.
F-26
Zen Healthcare
During the year ended December 31, 2020, we entered into a Collaboration Agreement, as amended and restated on August 4, 2021 (the “Zen Knightsbridge Collaboration Agreement”) with Purecare Limited (“Purecare”), a company that operates a health clinic known as Zen Knightsbridge Clinic (the “Zen Knightsbridge Clinic”), whereby both parties have agreed to collaborate on the provision of treatments at Purecare’s London based clinic. Additionally, during the year ended December 31, 2020, we entered into a Collaboration Agreement, as amended and restated on August 4, 2021 (the “Zen Baker Street Collaboration Agreement”) with Portman Health Ltd (“Portman”), a company that operates a health clinic known as Zen Baker Street Clinic (the “Zen Baker Street Clinic”).
Under
the Zen Knightsbridge Collaboration Agreement and the Zen Baker Street Collaboration Agreement, Purecare and Portman will provide consulting
and treatment rooms, apply for and maintain Care Quality Commission registrations, employ or engage licensed and qualified staff, assess
patients and, if appropriate, administer ketamine infusion treatments and any other treatments agreed to by the parties from time to
time (collectively, the “Treatments”), maintain equipment and provide all ketamine and other pharmaceuticals necessary for
the Treatments at the Zen Knightsbridge Clinic and the Zen Baker Street Clinic, respectively. Under the Zen Knightsbridge Collaboration
Agreement and the Zen Baker Street Collaboration Agreement, we agreed to, among other things, market the Treatments to the extent permitted
under law, arrange and pay for the fit-out of the consulting room, provide equipment necessary for the Treatments, develop, operate and
maintain a booking website for the Treatments, make bookings and take payments, and employ or engage customer services advisers to liaise
with clinical staff and pay certain staff costs. Under both the Zen Knightsbridge Collaboration Agreement and the Zen Baker Street Collaboration
Agreement, we were eligible to receive
Our
former Chief Operating Officer, Head of UK Clinics, Dr. Yassine Bendiabdallah, is a co-founder, current managing director, and
Brio Financial Group
On
April 13, 2021, we entered into the Brio Agreement pursuant to which Brio provided Stanley M. Gloss to serve as our Chief Financial Officer
and also provided certain other specified financial and accounting services typically provided by a chief financial officer. The term
of the Brio Agreement ran through March 31, 2022. The Company paid a monthly fixed fee of $
NOTE 15 – COMMITMENT AND CONTINGENCIES
Legal and Regulatory Environment
The healthcare industry is subject to numerous laws and regulations of federal, state and local governments. These laws and regulations include, but are not limited to, matters such as licensure, accreditation, government healthcare program participation requirement, reimbursement for patient services and Medicare and Medicaid fraud and abuse. Government activity has increased with respect to investigations and allegations concerning possible violations of fraud and abuse statutes and regulations by healthcare providers.
Violations of these laws and regulations could result in expulsion from government healthcare programs together
with the imposition of significant fines and penalties, as well as significant repayments for patient services previously billed. Management believes that the Company is in compliance with fraud and abuse regulations, as well as other applicable government laws and regulations. While no material regulatory inquiries have been made, compliance with such laws and regulations can be subject to future government review and interpretation, as well as regulatory actions unknown or unasserted at this time.
F-27
NOTE 16 – SUBSEQUENT EVENTS
The Company has evaluated events and transactions subsequent to December 31, 2022 through the date these consolidated financial statements were included on Form 10-K and filed with the SEC. Other than the below, there are no subsequent events identified that would require disclosure in these consolidated financial statements.
Nasdaq Deficiency Notice
On
January 19, 2023, the Company received a written notice (the “Notice”) from the Listing Qualifications Department of The
Nasdaq Stock Market (“Nasdaq”) indicating that the Company is not in compliance with the $
The
Nasdaq Listing Rules require listed securities to maintain a minimum bid price of $
Alternatively, if the Company fails to regain compliance with Rule 5550(a)(2) prior to the expiration of the initial 180 calendar day period, the Company may be eligible for an additional 180 calendar day compliance period, provided (i) it meets the continued listing requirement for market value of publicly held shares and all other applicable requirements for initial listing on The Nasdaq Capital Market (except for the Bid Price Requirement) and (ii) it provides written notice to Nasdaq of its intention to cure this deficiency during the second compliance period by effecting a reverse stock split, if necessary. In the event the Company does not regain compliance with Rule 5550(a)(2) prior to the expiration of the initial 180 calendar day period, and if it appears to the Staff that the Company will not be able to cure the deficiency, or if the Company is not otherwise eligible, the Staff will provide the Company with written notification that its securities are subject to delisting from The Nasdaq Capital Market. At that time, the Company may appeal the delisting determination to a hearings panel.
Issuance of Stock Options
On
February 24, 2023, the Company issued stock options under the 2021 Plan to purchase an aggregate of
F-28