
Filed Pursuant to Rule 433 Issuer
Free Writing Prospectus
dated August 26, 2021
File No. 333-255205

COPYRIGHT © 2021 PASITHEA Corporate Presentation 1

COPYRIGHT © 2021 PASITHEA FORWARD - LOOKING STATEMENTS This presentation shall not constitute an offer to sell or the solicitation of an offer to buy securities, nor shall there be an y sale of securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. This presentation contains forward - looking statements regarding future events and the future results of Pasithea Therapeutics Co rp. (“Pasithea” or the “Company”) that are based on current expectations, estimates, forecasts, and projections about the industry in which the Company operates and the beliefs and assumptions of the management of the Company. Words such as “address,” “anticipate,” “believe,” “consider,” “continue,” “develop,” “estimate,” “expect,” “further,” “goal,” “intend,” “may,” “plan,” “potential,” “project,” “seek,” “should,” “targe t,” “will,” variations of such words, and similar expressions are intended to identify such forward - looking statements. Such statements reflect the current views of the Company and its management with respect to future events an d are subject to certain risks, uncertainties, and assumptions. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, the Company’s actual results, per for mance, or achievements could differ materially from the results expressed in, or implied by, these forward - looking statements. This presentation has been prepared by the Company based on information it has obtained from s ources it believes to be reliable. Summaries of documents contained in this presentation may not be complete. This presentation contains industry, market and competitive position data from our own internal estimate s a nd research as well as industry and general publications and research surveys and studies conducted by third parties. Industry publications, studies and surveys generally state that they have been obtained from sour ces believed to be reliable, although they do not guarantee the accuracy or completeness of such information. Our internal data and estimates are based upon information obtained from trade and business organizations and ot her contacts in the markets in which we operate and our management’s understanding of industry conditions. While we believe that each of these studies and publications is reliable, we have not independently veri fie d market and industry data from third - party sources. While we believe our internal company research is reliable and the market definitions are appropriate, neither such research nor definitions have been verified by an independent source. The Company does not represent that the information herein is complete. The information in this presentation is current only as of August 2021, and the Company’s business or financial condition and ot her information in this presentation may change after that date. The Company disclaims any obligation to publicly update or release any revisions to the forward - looking information contained in this presentation, whethe r as a result of new information, future events or otherwise, after the date of this presentation or to reflect the occurrence of unanticipated events, except as required by law. This presentation contains projected financial information with respect to the Company. Such projected financial information con stitutes forward - looking information, and is for illustrative purposes only and should not be relied upon as necessarily being indicative of future results. The assumptions and estimates underlying such projected financ ial information are inherently uncertain and are subject to a wide variety of significant business, economic, competitive and other risks and uncertainties that could cause actual results to differ materially from those conta ine d in the prospective financial information. Actual results may differ materially from the results contemplated by the projected financial information contained in this presentation, and the inclusion of such information in thi s presentation should not be regarded as a representation by any person that the results reflected in such projections will be achieved. This presentation may not be copied or reproduced in whole or in part. By accepting delivery of this presentation, you agree to these restrictions. We have filed a registration statement (including a preliminary prospectus) with the SEC for the offering to which this commu nic ation relates. The registration statement has not yet become effective. Before you invest, you should read the preliminary prospectus in that registration statement (including the risk factors described therein) and othe r d ocuments that we have filed with the SEC for more complete information about us and the offering. We encourage you to read the registration statement and the prospectus in full for more detailed information on the st atistics, reports, studies, and clinical trials references in this presentation. You may access these documents for free by visiting EDGAR on the SEC Website at http://www.sec.gov . Alternatively, we or any underwriter participating in this offering will arrange to send you the prospectus if you contact Dr. Tiago Reis Marques, Chief Executive Officer, at (702) 514 - 4174, or EF Hutton, division of Benchmark Investments, LLC , 590 Madison Ave., 39 th Floor, New York, NY 10022, Attention: Syndicate Department, or via email at syndicategroup@efhuttongroup.com or telephone at (212) 404 - 7002. 2
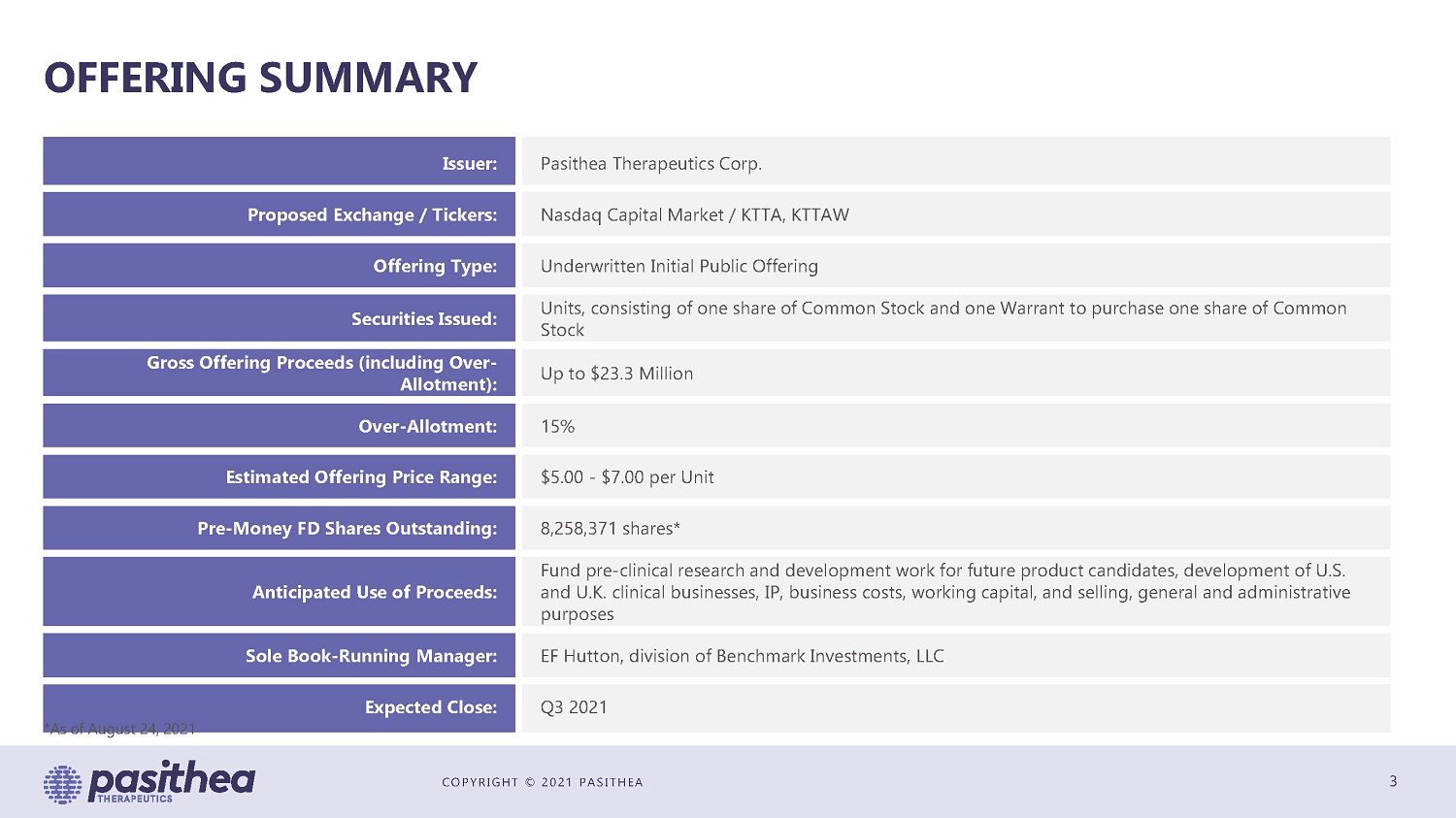
COPYRIGHT © 2021 PASITHEA OFFERING SUMMARY Issuer: Proposed Exchange / Tickers: Offering Type: Securities Issued: Gross Offering Proceeds (including Over- Allotment): Over-Allotment: Estimated Offering Price Range: Pre-Money FD Shares Outstanding: Anticipated Use of Proceeds: Sole Book-Running Manager: Expected Close: *As of August 24, 2021 Pasithea Therapeutics Corp. Nasdaq Capital Market / KTTA, KTTAW Underwritten Initial Public Offering Units, consisting of one share of Common Stock and one Warrant to purchase one share of Common Stock Up to $23.3 Million 15% $5.00 - $7.00 per Unit 8,258,371 shares* Fund pre-clinical research and development work for future product candidates, development of U.S. and U.K. clinical businesses, IP, business costs, working capital, and selling, general and administrative purposes EF Hutton, division of Benchmark Investments, LLC Q3 2021
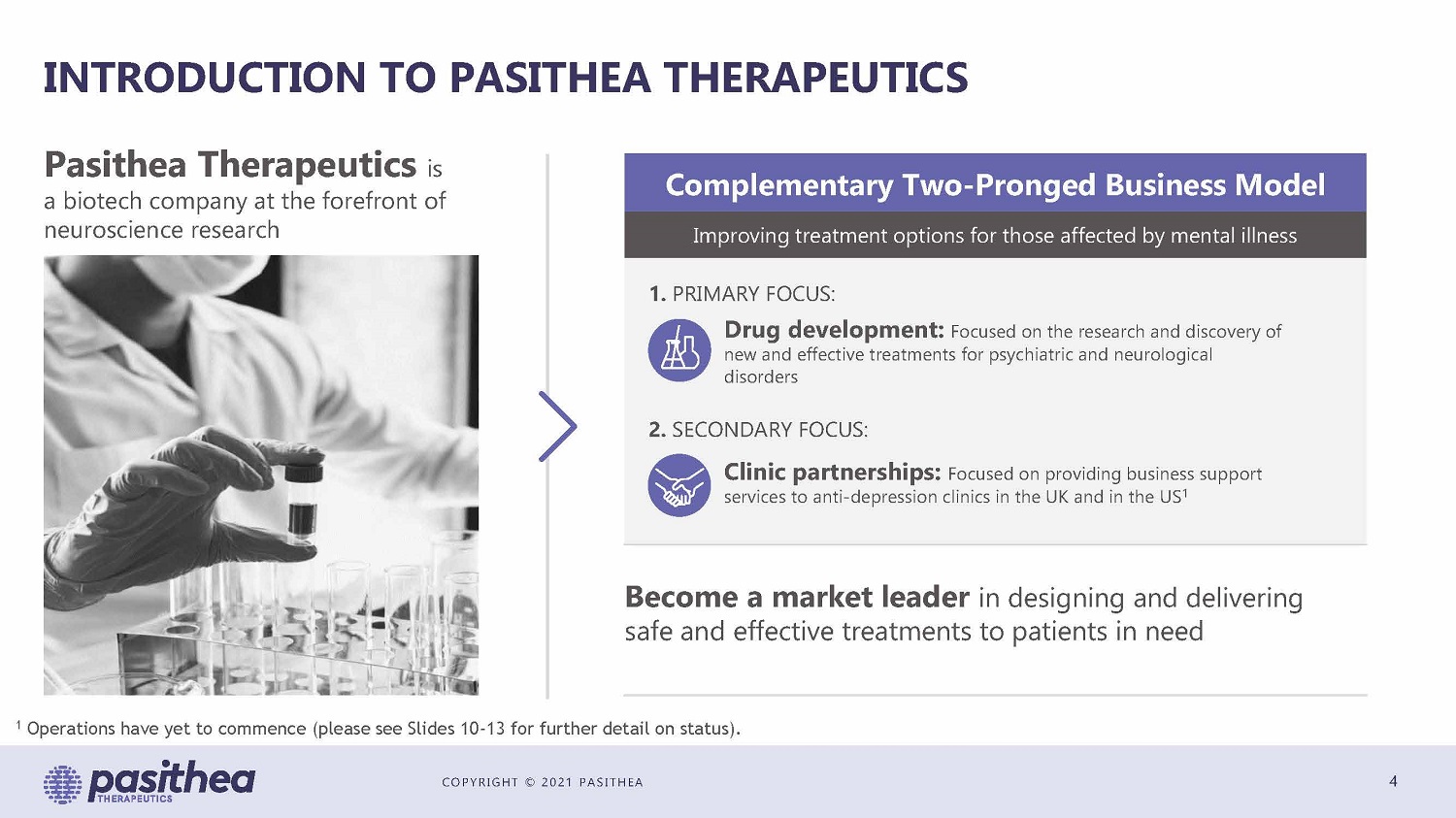
COPYRIGHT © 2021 PASITHEA INTRODUCTION TO PASITHEA THERAPEUTICS 4 Pasithea Therapeutics is a biotech company at the forefront of neuroscience research Drug development: Focused on the research and discovery of new and effective treatments for psychiatric and neurological disorders Clinic partnerships: Focused on providing business support services to anti - depression clinics in the UK and in the US 1 Complementary Two - Pronged Business Model Improving treatment options for those affected by mental illness Become a market leader in designing and delivering safe and effective treatments to patients in need 1. PRIMARY FOCUS: 2. SECONDARY FOCUS: 1 Operations have yet to commence (please see Slides 10 - 13 for further detail on status).

COPYRIGHT © 2021 PASITHEA INVESTMENT HIGHLIGHTS 5 Large Addressable Market With Few Options for Adequate Help Today Capital - Light Infrastructure Diversified Revenue Stream with Significant Revenue and EBITDA Growth Potential Seasoned Leadership Team with Expertise in Neuroscience & Psychopharmacology Dr. Lawrence Steinman, Executive Chairman & Co - Founder • Endowed Chair in the Neurology Dept. at Stanford University • Founded several successful biotech companies • Drug development pioneer Dr. Tiago Reis Marques, CEO & Director • Fellow at Imperial College and lecturer at King s College London • Renowned psychiatric researcher and lecturer with decades of experience in the biological mechanisms of mental health and brain disorders Dr. Yassine Bendiabdallah, COO, Head of UK Clinics & Director • PhD in Medicinal Chemistry from University College London • Extensive experience in the design and synthesis of novel drug candidates
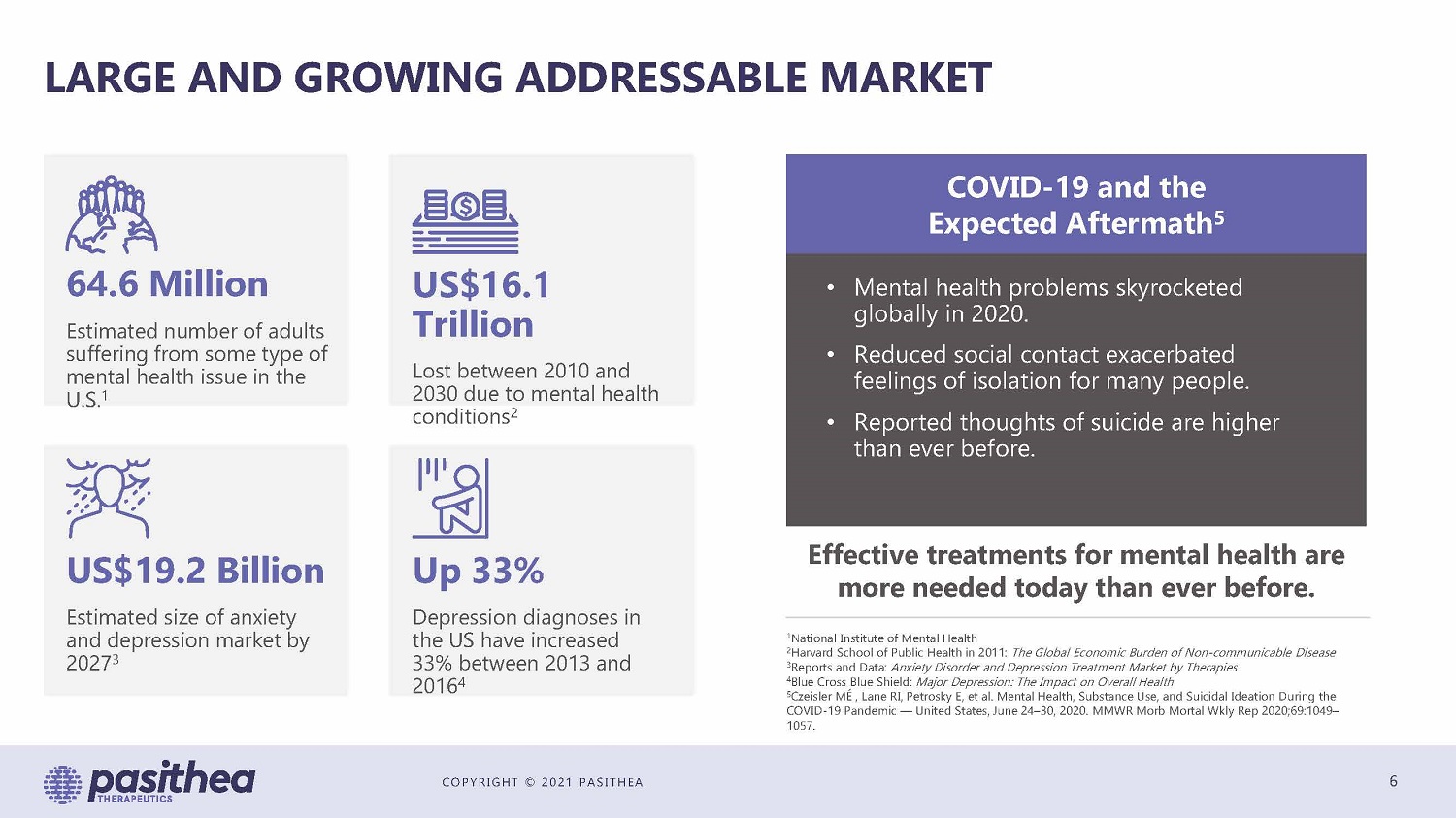
COPYRIGHT © 2021 PASITHEA LARGE AND GROWING ADDRESSABLE MARKET 6 64.6 Million Estimated number of adults suffering from some type of mental health issue in the U.S. 1 US$16.1 Trillion Lost between 2010 and 2030 due to mental health conditions 2 US$19.2 Billion Estimated size of anxiety and depression market by 2027 3 Up 33% Depression diagnoses in the US have increased 33% between 2013 and 2016 4 COVID - 19 and the Expected Aftermath 5 • Mental health problems skyrocketed globally in 2020. • Reduced social contact exacerbated feelings of isolation for many people. • Reported thoughts of suicide are higher than ever before. Effective treatments for mental health are more needed today than ever before. 1 National Institute of Mental Health 2 Harvard School of Public Health in 2011: The Global Economic Burden of Non - communicable Disease 3 Reports and Data: Anxiety Disorder and Depression Treatment Market by Therapies 4 Blue Cross Blue Shield: Major Depression: The Impact on Overall Health 5 Czeisler MÉ , Lane RI, Petrosky E, et al. Mental Health, Substance Use, and Suicidal Ideation During the COVID - 19 Pandemic — United States, June 24 – 30, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1049 – 1057.
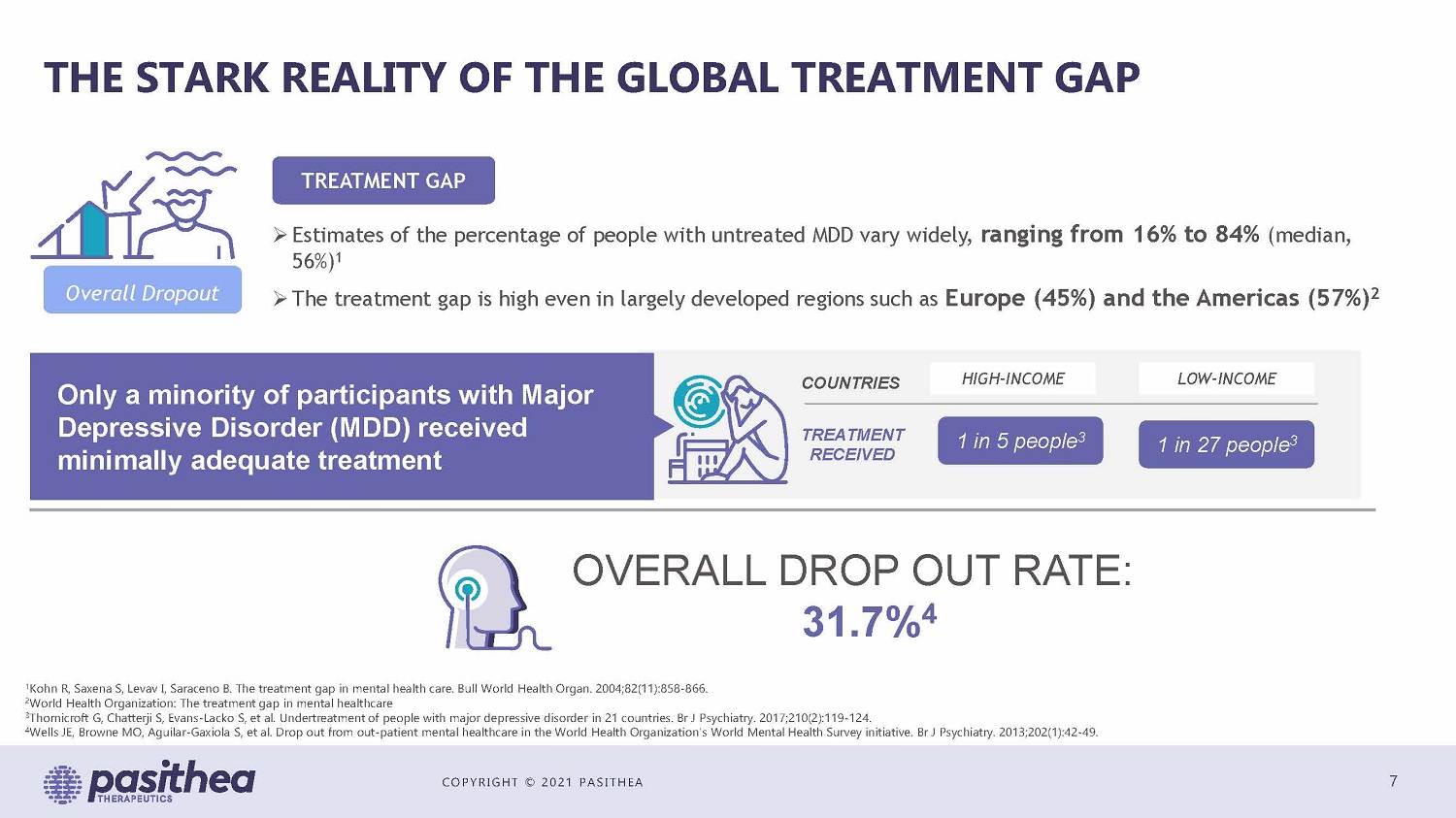
COPYRIGHT © 2021 PASITHEA THE STARK REALITY OF THE GLOBAL TREATMENT GAP 7 Overall Dropout Only a minority of participants with MDD received minimally adequate treatment HIGH - INCOME LOW - INCOME 1 in 5 people 3 1 in 27 people 3 COUNTRIES TREATMENT RECEIVED OVERALL DROP OUT RATE: 31.7% 4 » Estimates of the percentage of people with untreated MDD vary widely, ranging from 16% to 84% (median, 56%) 1 » The treatment gap is high even in largely developed regions such as Europe (45%) and the Americas (57%) 2 TREATMENT GAP 1 Kohn R, Saxena S, Levav I, Saraceno B. The treatment gap in mental health care. Bull World Health Organ. 2004;82(11):858 - 866. 2 World Health Organization: The treatment gap in mental healthcare 3 Thornicroft G, Chatterji S, Evans - Lacko S, et al. Undertreatment of people with major depressive disorder in 21 countries. Br J Psychiatry. 2017;210(2):119 - 124. 4 Wells JE, Browne MO, Aguilar - Gaxiola S, et al. Drop out from out - patient mental healthcare in the World Health Organization's Wo rld Mental Health Survey initiative. Br J Psychiatry. 2013;202(1):42 - 49. Only a minority of participants with Major Depressive Disorder (MDD) received minimally adequate treatment
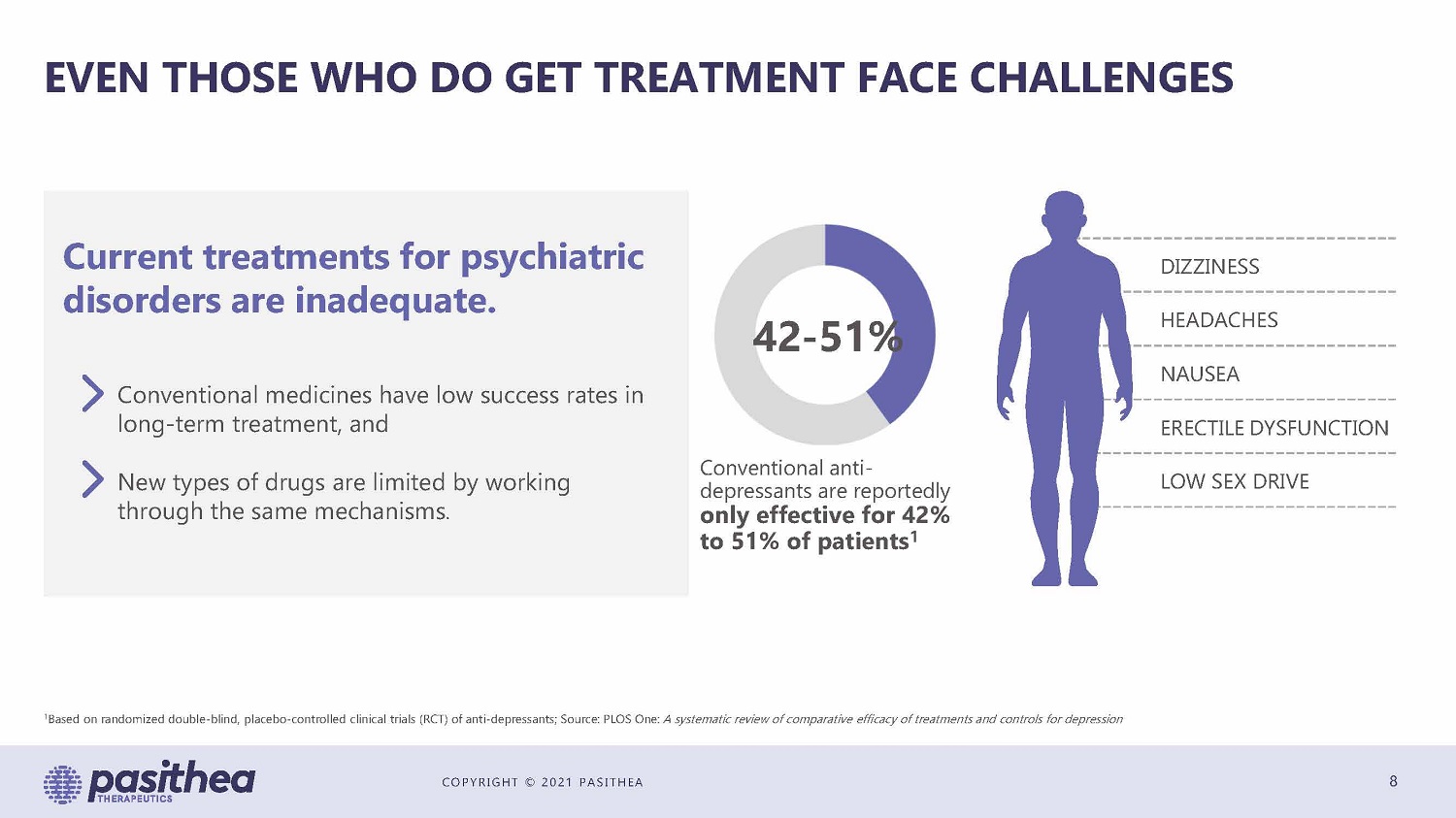
COPYRIGHT © 2021 PASITHEA EVEN THOSE WHO DO GET TREATMENT FACE CHALLENGES 8 Current treatments for psychiatric disorders are inadequate. Conventional medicines have low success rates in long - term treatment, and New types of drugs are limited by working through the same mechanisms . DIZZINESS HEADACHES NAUSEA ERECTILE DYSFUNCTION LOW SEX DRIVE 42 - 51% Conventional anti - depressants are reportedly only effective for 42% to 51% of patients 1 1 Based on randomized double - blind, placebo - controlled clinical trials (RCT) of anti - depressants; Source: PLOS One: A systematic review of comparative efficacy of treatments and controls for depression
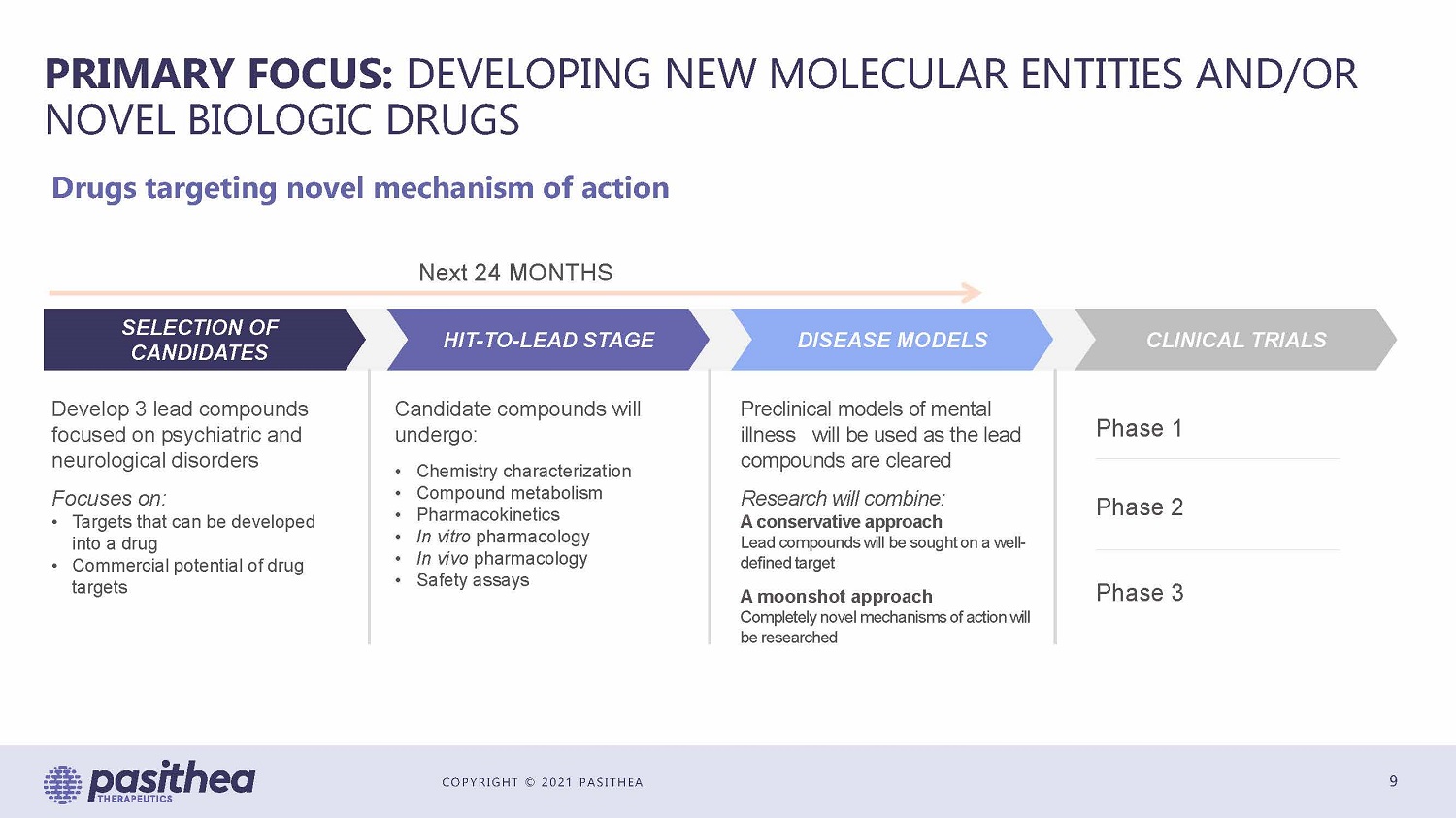
COPYRIGHT © 2021 PASITHEA PRIMARY FOCUS: DEVELOPING NEW MOLECULAR ENTITIES AND/OR NOVEL BIOLOGIC DRUGS 9 Drugs targeting novel mechanism of action Next 24 MONTHS Phase 1 Phase 2 Phase 3 Develop 3 lead compounds focused on psychiatric and neurological disorders Focuses on: • Targets that can be developed into a drug • Commercial potential of drug targets Candidate compounds will undergo: • Chemistry characterization • Compound metabolism • Pharmacokinetics • In vitro pharmacology • In vivo pharmacology • Safety assays Preclinical models of mental illness will be used as the lead compounds are cleared Research will combine: A conservative approach Lead compounds will be sought on a well - defined target A moonshot approach Completely novel mechanisms of action will be researched SELECTION OF CANDIDATES HIT - TO - LEAD STAGE DISEASE MODELS CLINICAL TRIALS
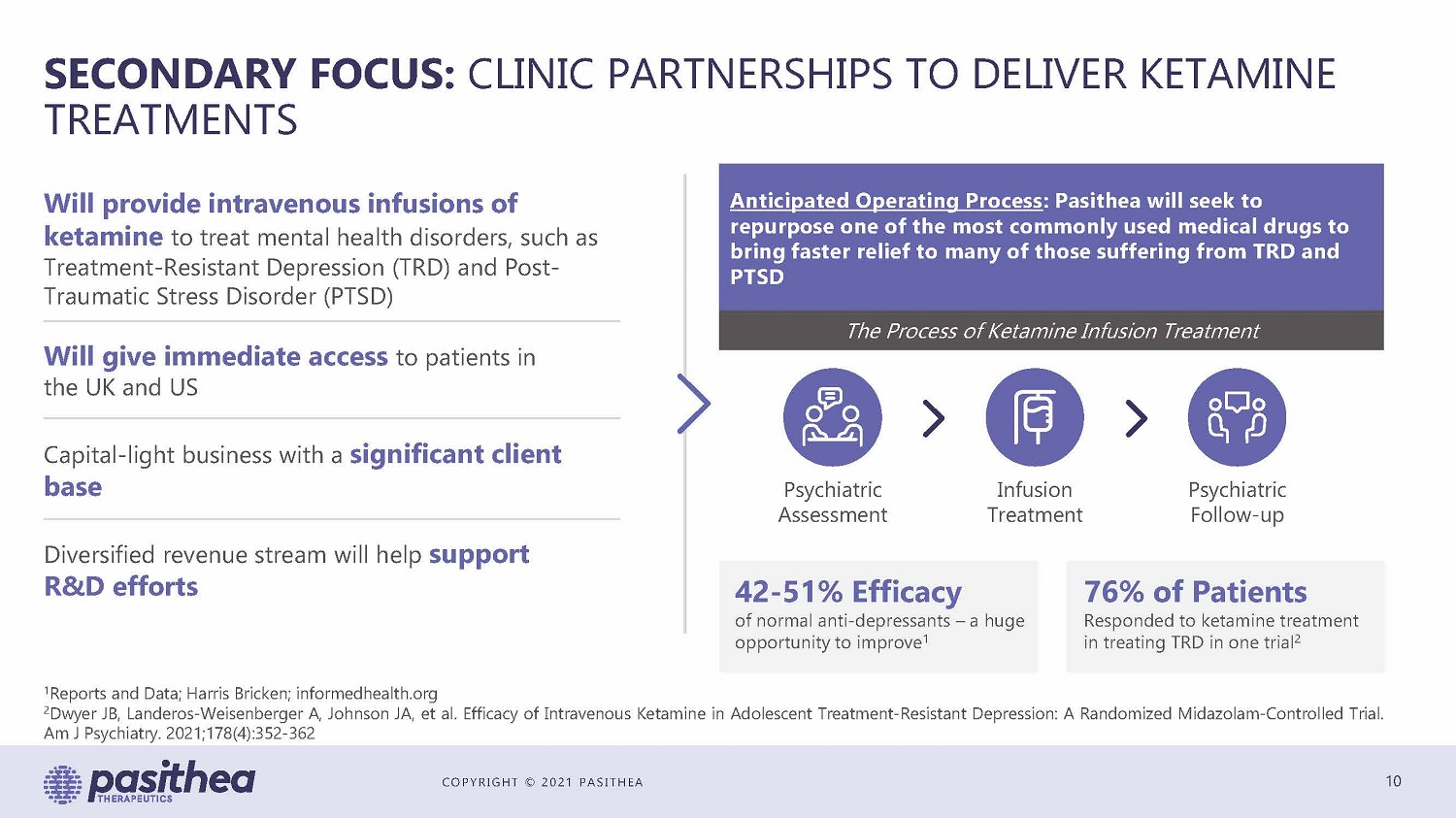
COPYRIGHT © 2021 PASITHEA SECONDARY FOCUS: CLINIC PARTNERSHIPS TO DELIVER KETAMINE TREATMENTS 10 Will provide intravenous infusions of ketamine to treat mental health disorders, such as Treatment - Resistant Depression (TRD) and Post - Traumatic Stress Disorder (PTSD) Will give immediate access to patients in the UK and US Capital - light business with a significant client base Diversified revenue stream will help support R&D efforts Anticipated Operating Process : Pasithea will seek to repurpose one of the most commonly used medical drugs to bring faster relief to many of those suffering from TRD and PTSD The Process of Ketamine Infusion Treatment Psychiatric Assessment Infusion Treatment Psychiatric Follow - up 42 - 51% Efficacy of normal anti - depressants – a huge opportunity to improve 1 76% of Patients Responded to ketamine treatment in treating TRD in one trial 2 1 Reports and Data ; Harris Bricken ; informedhealth . org 2 Dwyer JB, Landeros - Weisenberger A, Johnson JA, et al . Efficacy of Intravenous Ketamine in Adolescent Treatment - Resistant Depression : A Randomized Midazolam - Controlled Trial . Am J Psychiatry . 2021 ; 178 ( 4 ) : 352 - 362

COPYRIGHT © 2021 PASITHEA SECONDARY FOCUS: CLINIC PARTNERSHIPS TO DELIVER KETAMINE TREATMENTS – UNITED KINGDOM 11 Operations in the UK will involve providing business support services to registered healthcare providers who will assess pati ent s and administer IV infusion of ketamine, if appropriate. Operations in the UK will take placement through partnerships with Zen Healthcare . Collaboration Agreements with Purecare Limited and Portman Health Ltd • Pasithea obligations — Market treatments to extent permitted by law — Arrange and pay for fit - out of consulting room — Provide equipment — Employ/engage customer services advisors to liaise with clinical staff and pay certain staff costs — Operate and maintain booking website for treatments and take bookings and payment • Purecare Limited and Portman Health Ltd obligations — Apply for and maintain CQC registrations — Provide consulting and treatment rooms and maintain equipment — Provide pharmaceuticals and equipment for assessing patients and providing treatments — Employ/engage licensed and qualified staff — Assess patients and administer treatment, if appropriate Currently, Pasithea Therapeutics Limited (UK) currently has one employee and will operate under Zen Healthcare’s regulatory a ppr ovals.
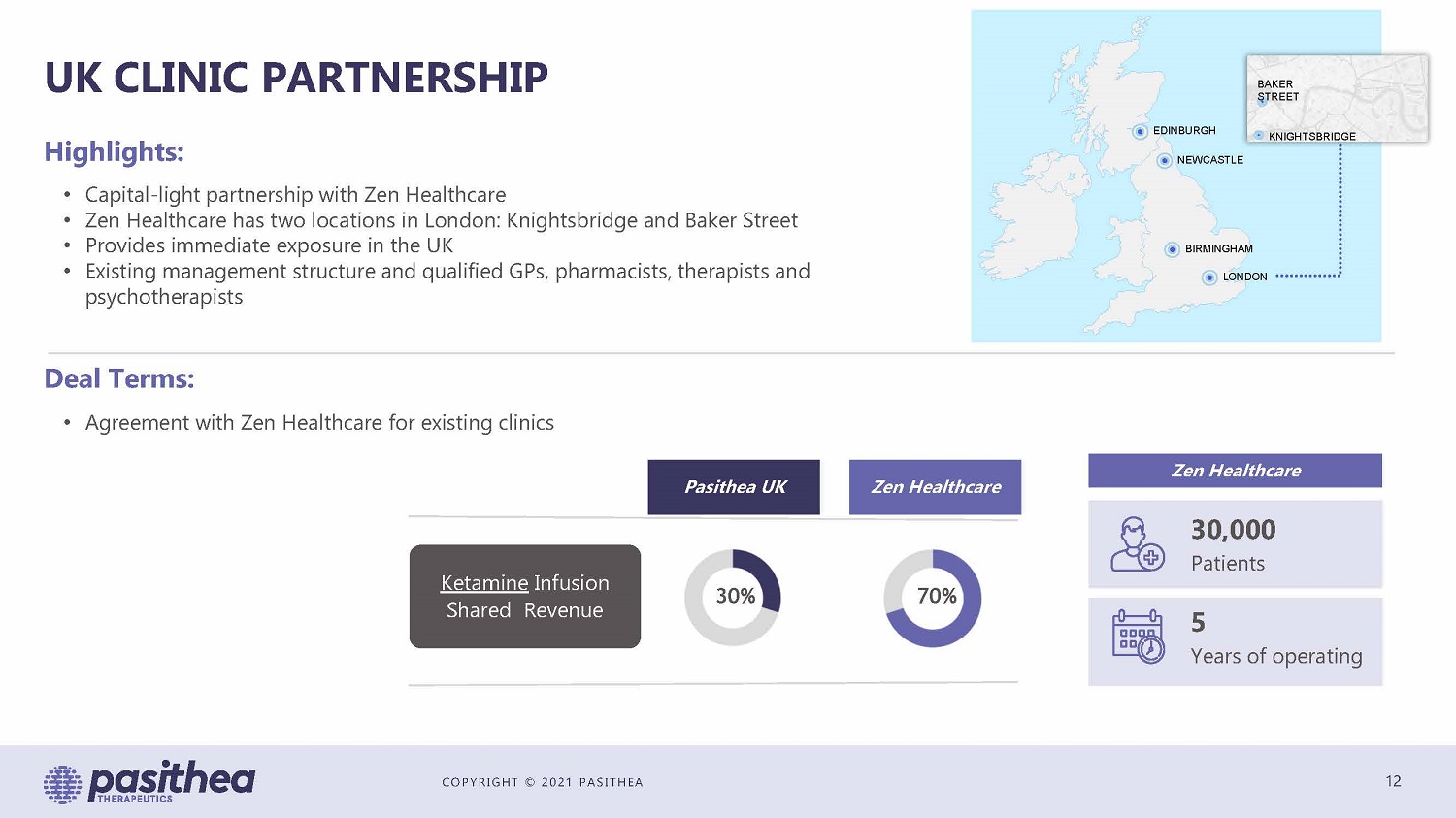
COPYRIGHT © 2021 PASITHEA UK CLINIC PARTNERSHIP 12 • Capital - light partnership with Zen Healthcare • Zen Healthcare has two locations in London: Knightsbridge and Baker Street • Provides immediate exposure in the UK • Existing management structure and qualified GPs, pharmacists, therapists and psychotherapists Highlights: Deal Terms: • Agreement with Zen Healthcare for existing clinics Zen Healthcare 30,000 Patients 5 Years of operating 70% 30% Zen Healthcare Pasithea UK Ketamine Infusion Shared Revenue EDINBURGH NEWCASTLE BIRMINGHAM LONDON BAKER STREET KNIGHTSBRIDGE

COPYRIGHT © 2021 PASITHEA SECONDARY FOCUS: CLINIC PARTNERSHIPS TO DELIVER KETAMINE TREATMENTS –UNITED STATES Operations in the US will involve providing business support services to registered healthcare providers who will assess patients and administer IV infusion of ketamine, if appropriate. Pasithea intends to enter into Business Support Services Agreements with professional companies, Nadelson Medical PLLC and Nadelson Medical of CA, P.C. •Nadelson Medical PLLC is currently being formed •Pasithea expects to have executed Business Support Services Agreements with both professional companies by September 2021 Pasithea has entered into a Business Support Services Subcontract with The IV Doc. •The IV Doc will provide certain non-clinical administrative, back office, and other business support services to one or more professional medical practices in New York.•Pasithea will pay The IV Doc monthly subcontract fees and reimburse The IV Doc for all reasonable expenses. 13
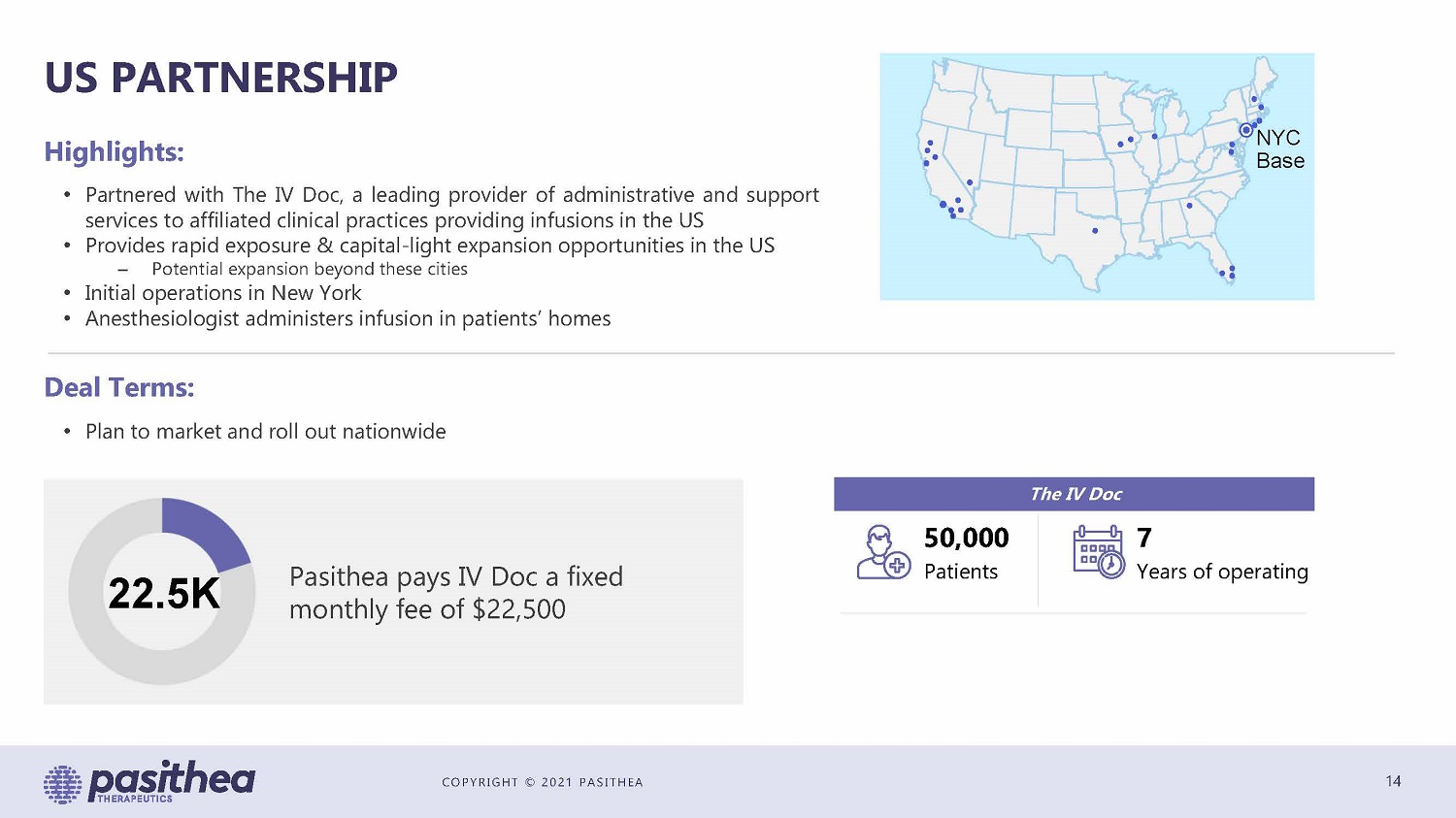
COPYRIGHT © 2021 PASITHEA US PARTNERSHIP 14 NYC Base Deal Terms: • Plan to market and roll out nationwide 22.5K Pasithea pays IV Doc a fixed monthly fee of $22,500 The IV Doc 50,000 Patients 7 Years of operating • Partnered with The IV Doc, a leading provider of administrative and support services to affiliated clinical practices providing infusions in the US • Provides rapid exposure & capital - light expansion opportunities in the US – Potential expansion beyond these cities • Initial operations in New York • Anesthesiologist administers infusion in patients’ homes Highlights:

COPYRIGHT © 2021 PASITHEA PASITHEA’S COMPETITIVE LANDSCAPE IN THE MENTAL HEALTH SECTOR 15
COPYRIGHT © 2021 PASITHEA IMPROVING MENTAL HEALTH TREATMENTS AND DRIVING SHAREHOLDER VALUE 16 Seasoned Leadership Team with Expertise in Neuroscience & Psychopharmacology Diversified Revenue Stream with Significant Revenue and EBITDA Growth Potential Capital - Light Infrastructure Large Addressable Market With Few Options for Adequate Help Today Drug development: Focused on the research and discovery of new and effective treatments for psychiatric and neurological disorders Clinic partnerships: Focused on providing business support services to anti - depression clinics in the UK and in the US 1 Complementary Two - Pronged Business Model Improving treatment options for those affected by mental illness Become a market leader in designing and delivering safe and effective treatments to patients in need 1. PRIMARY FOCUS: 2. SECONDARY FOCUS: 1 Operations have yet to commence (please see Slides 10 - 13 for further detail on status).

COPYRIGHT © 2021 PASITHEA 17 www.pasitheaclinics.com Contact: Ed Tsuker Head of Capital Markets EF Hutton etsuker@efhuttongroup.com