
As filed with the Securities and Exchange Commission on April 13, 2021.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Pasithea Therapeutics Corp.
(Exact name of registrant as specified in its charter)
| Delaware | 2834 | 85-1591963 | ||
| (State
or other jurisdiction of incorporation or organization) |
(Primary
Standard Industrial Classification Code Number) |
(I.R.S.
Employer Identification No.) |
1111 Lincoln Road
Suite 500
Miami Beach, FL 33139
702-514-4174
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Dr. Tiago Reis Marques
Chief Executive Officer
Pasithea Therapeutics Corp.
1111 Lincoln Road
Suite 500
Miami Beach, FL 33139
702-514-4174
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
Robert Cohen Richard Bass McDermott Will & Emery LLP 340 Madison Avenue New York, NY 10173-1922 Telephone: (212) 547-5885 |
Richard Friedman Stephen Cohen Nazia Khan Sheppard, Mullin, Richter & Hampton LLP 30 Rockefeller Plaza New York, NY 10112-0015 Telephone: (212) 653-8700 |
Approximate date of commencement of proposed sale to the public:
As soon as practicable after this Registration Statement is declared effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| Emerging growth company | ☒ |
If an emerging growth company, indicate by checkmark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities To Be Registered | Proposed Maximum Aggregate Offering Price(1)(2) | Amount
of Registration Fee(3) | ||||||
| Common Stock, $0.0001 par value per share | $ | 20,000,000 | $ | 2,182 | ||||
| (1) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended. |
| (2) | Includes the aggregate offering price of additional shares of common stock that the underwriters have the option to purchase to cover over-allotments, if any. |
| (3) | Calculated pursuant to Rule 457(o) based on an estimate of the proposed maximum aggregate offering price. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED APRIL 13, 2021
PRELIMINARY PROSPECTUS
Shares

Pasithea Therapeutics Corp.
Common Stock
We are offering shares of our common stock (“Common Stock”). This is our initial public offering. Prior to the offering, there has been no public market for our Common Stock. We expect the initial public offering price to be between $ and $ per share. We intend to apply to list our Common Stock on The Nasdaq Capital Market under the symbol “KTTA”.
We are an “emerging growth company” under the federal securities laws and, as such, we have elected to comply with certain reduced public company reporting requirements for this prospectus and future filings. See “Prospectus Summary—Implications of Being an Emerging Growth Company and a Smaller Reporting Company.”
Investing in our Common Stock involves a high degree of risk. Before buying any shares, you should carefully read the discussion of the material risks of investing in our Common Stock under the heading “Risk Factors” beginning on page 15 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed on the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
| Per share | Total | |||||||
| Public offering price | $ | $ | ||||||
| Underwriting discounts and commissions (1) | $ | $ | ||||||
| Proceeds, before expenses, to us | $ | $ | ||||||
| (1) | See “Underwriters” beginning on page 103 of this prospectus for additional information regarding the compensation payable to the underwriters. |
We have granted a 45-day option to the underwriters to purchase up to additional shares of Common Stock solely to cover over-allotments, if any. If the underwriters exercise the option in full, the total underwriting discounts and commissions payable by us will be $ , and the total proceeds to us, before expenses, will be $ .
Delivery of the shares of Common Stock is expected to be made on or about , 2021.
KINGSWOOD CAPITAL MARKETS
division of Benchmark Investments, Inc.
The date of this prospectus is , 2021
Neither we nor the underwriters have authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectus prepared by or on behalf of us or to which we have referred you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the shares of Common Stock offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus or in any applicable free writing prospectus is current only as of its date, regardless of its time of delivery or any sale of shares of our Common Stock. Our business, financial condition, results of operations and prospects may have changed since that date.
For investors outside the United States: Neither we nor the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of Common Stock and the distribution of this prospectus outside the United States.
FINANCIAL STATEMENT PRESENTATION
The consolidated financial statements as of December 31, 2020 and for the period May 12, 2020 (inception) to December 31, 2020 represent the operations of Pasithea Therapeutics Corp. and its wholly owned subsidiaries, Pasithea Therapeutics Limited (UK) and Pasithea Clinics Inc. All inter-company balances and transactions among the companies have been eliminated upon consolidation.
i
ABOUT THIS PROSPECTUS
Except where the context otherwise requires or where otherwise indicated throughout this registration statement, the terms “Pasithea,” “we,” “us,” our,” “our company,” “Company” and “our business” refer to Pasithea Therapeutics Corp. and its wholly owned subsidiaries, Pasithea Therapeutics Limited (UK) and Pasithea Clinics Inc.
ii
This summary highlights, and is qualified in its entirety by, the more detailed information and financial statements included elsewhere in this prospectus. This summary does not contain all of the information that may be important to you in making your investment decision. You should read this entire prospectus carefully, especially the “Risk Factors” section beginning on page 15 and our financial statements and the related notes included elsewhere in this prospectus, before making an investment decision.
Business Overview
We are a biotechnology company focused on the research and discovery of new and effective treatments for psychiatric and neurological disorders. Epidemiological data indicate neuropsychiatric disorders as being some of the most prevalent, devastating, and yet poorly treated illnesses. We believe that the current treatments for these disorders, such as depression, are inadequate and that conventional medicines have low success rates in long-term treatment. For example, current pharmacotherapies for major depressive disorder (MDD) and bipolar depression (BDep) have a distinct lag of onset that can generate further distress and impairment in patients. Traditional psychiatric drugs can also cause serious side effects. Furthermore, the approval of psychotropic drugs with novel mechanisms of action has been rare in recent years. Our biotech operations will focus on developing drugs that target the pathophysiology underlying such disorders rather than symptomatic treatments, with the goal of developing new pharmacological agents that display significant advantages over conventional therapies with respect to efficacy and tolerability. We will particularly focus on the cross-talk between the immune system and brain disorders and how immune dysregulation affects central nervous system (CNS) function. Our drug discovery efforts will focus on neuropsychiatric disorders that although phenotypically distinct are pathogenically related. We aim to focus on mechanism-based immune treatments for the treatment of these disorders.
Our secondary operations are focused on establishing anti-depression clinics across the United Kingdom and providing business support services to similar entities in the United States and using psychiatric assessment combined with physician/medical providers to administer intravenous infusions of ketamine. Operations will initially take place across the United States and the United Kingdom through partnerships with healthcare companies, including with Zen Healthcare and The IV Doc Inc. (“The IV Doc”).
Ketamine was first introduced to the medical community as a surgical anesthetic more than 50 years ago. According to “Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review,” a 2015 article published by Therapeutic Advances in Chronic Disease, a peer-reviewed open access journal, and “Ketamine for major depression: New tool, new questions,” a 2019 article published on the Harvard Medical School’s website, as of the date of this prospectus, ketamine is gaining grounds as a promising treatment for some cases of major depression. It works differently than traditional antidepressants, which target the brain’s serotonin and noradrenalin systems. Ketamine blocks N-methyl-D-aspartate (NMDA), a receptor in the brain that is activated by glutamate, a neurotransmitter. A single subanesthetic dose infusion of the NMDA receptor antagonist ketamine has been shown to have potentially rapid and potent antidepressant effects in treatment-resistant MDD as well as for the treatment of post-traumatic stress disorder. While not approved by the U.S. Food and Drug Administration (FDA) or the Medicines and Healthcare products Regulatory Agency (MHRA) to treat depression, and while recreational use remains prohibited, subject to appropriate caution and review, doctors and pharmacists prescribe ketamine for medical purposes, a practice endorsed by the American Psychiatric Association. Ketamine’s potential safety and effectiveness have been demonstrated in multiple research studies. As many as 70% of those who get ketamine infusions show a response, typically after the first session. If a person responds to ketamine, it may rapidly reduce suicidality and relieve other symptoms of depression.
As of March 26, 2021, the Company had not commenced core operations. All activity for the period from May 12, 2020 (inception) through March 26, 2021 relates to the Company’s formation and raising funds through issuing shares of the Company’s Common Stock. The Company has selected December 31 as its fiscal year end.
1
Our Strategy
Our core strategy is to become a leader in solving psychiatric and neurological disorders, one of the world’s biggest clinical problems, through research, development, and commercialization of novel CNS drugs. Key elements of our business strategy are as follows:
| ● | Research new drugs with different mechanism of action to conventional psychiatric and neurological drugs, targeting the pathophysiology underlying the disease, for the treatment of CNS disorders under the leadership of Professor Lawrence Steinman, a renowned neurologist and immunologist based at Stanford University, and Dr. Tiago Reis Marques, a psychiatrist and neuroscientist at Imperial College and King’s College London; | |
| ● | Partner with reputable and successful healthcare companies and clinics to provide and support the intravenous administration of ketamine to treat treatment-resistant depression; |
| ○ | Create a capital efficient revenue stream with significant client bases across the United States and the United Kingdom, including in Los Angeles, New York City, and London; and | |
| ○ | Create a diversified revenue stream by establishing and supporting clinics to provide greater visibility of revenue and EBITDA. |
Development Pipeline
Our current research plan, which is aimed at developing new molecular entities and/or novel biologic drugs in the 24 months following the closing of this offering, is as follows:
1. Selection of Candidates. We plan to select and develop three lead candidate compounds focused on the neurobiology of psychiatric and neurological disorders that can be developed into drugs and which have commercial potential as drug targets.
2. Hit to Lead Stage. Next, we plan to put the candidate compounds through a hit to lead stage, which is a stage in early drug discovery where small molecule hits from a high throughput screen are evaluated and undergo limited optimization to identify promising lead compounds. The candidate compounds will undergo chemistry characterization, compound metabolism, pharmacokinetics, in vitro pharmacology, in vivo pharmacology, and safety assays.
3. Disease Models. We plan to use preclinical models of psychiatric and neurological disorders, as the lead compounds are cleared. Our research will combine a conservative approach, under which lead compounds will be sought on a well-defined target, and a moonshot approach, under which completely novel mechanisms of action will be researched.
After 24 months, and after we develop one or more product candidates, subject to FDA and other similar regulatory approvals, we aim to begin one or more clinical trials.
About Our Indications
According to the National Institute of Mental Health, mental illnesses are common in the United States. Mental illnesses include many different conditions that vary in degree of severity, ranging from mild to moderate to severe. Two broad categories can be used to describe these conditions: Any Mental Illness (AMI) and Serious Mental Illness (SMI). AMI encompasses all recognized mental illnesses, whereas SMI is a smaller and more severe subset of AMI.
In 2019, there were an estimated 51.5 million adults aged 18 or older in the United States with AMI. Among the 51.5 million adults with AMI, 23.0 million (44.8%) received mental health services in the past year. In 2019, there were an estimated 13.1 million adults aged 18 or older in the United States with SMI, which represented 5.2% of all U.S. adults. Out of the 13.1 million adults with SMI, 8.6 million (65.5%) received mental health treatment in the past year.
According to the Mayo Clinic, treatment for mental illness largely depends on the type of mental illness and its severity. Currently, treatment can include psychiatric medication (such as anti-depressants, anti-anxiety medications, mood stabilizers, and antipsychotic drugs), psychotherapy, brain-stimulation treatments, hospitalization, substance misuse treatment, or any combination of the foregoing.
2
Clinical Services
Our secondary operations are focused on establishing anti-depression clinics across the United Kingdom and providing business support services to similar entities in the United States, using psychiatric assessment combined with physician/medical providers to provide private intravenous infusions of ketamine to treat treatment-resistant depression through partnerships with healthcare companies including with Zen Healthcare and The IV Doc.
United Kingdom. Pasithea’s United Kingdom branch has already partnered with Zen Healthcare, a general practice group with three locations: Marylebone, Knightsbridge, and Holborn. Zen Healthcare has been operating for five years and has approximately 30,000 patients. Its practices give us immediate exposure in the United Kingdom. Other advantages including gaining access to an existing management structure and qualified general practitioners, pharmacists, therapists, and psychotherapists. In the future, we plan to open independent clinics in London and other top regional cities in the United Kingdom.
In the United Kingdom (UK), Pasithea Therapeutics Corp. has established a wholly owned subsidiary organized under United Kingdom Law to provide psychotherapy and to administer IV ketamine in clinics. Under the laws of the UK, this entity may directly own and operate clinics, employ physicians, and provide management services to clinics and providers. In order to do so, the UK entity must obtain approvals from the following agencies: MHRA, Care Quality Commission (CQC), General Medical Council (GMC) and the General Pharmaceutical Council (GPC).
Specifically, in the UK, Pasithea will be responsible for obligations such as maintaining a CQC license, marketing ketamine and other treatments, booking and taking payments from patients, providing licensed and qualified staff and all pharmaceuticals and equipment necessary for the assessment of patients and provision of the treatments, assessing patients, and administering treatments. At the present, Pasithea has partnered with Purecare Limited and Portman Health Ltd that own Zen clinics to treat patients, including providing psychiatric consultations, and that have pharmacies that will procure, handle, and administer ketamine in treatment rooms, providing all pharmaceuticals and equipment necessary for the assessment of patients and the provision of the treatments.
In the UK, ketamine is a Schedule II controlled substance under the Misuse of Drugs Regulations 2010 and is controlled with regard to synthesis, storage and distribution under the Misuse of Drugs Act 1971 as amended. Possession of ketamine requires Home Office licensing and may only be stored on premises complying with professional strictures of the GPC. As a controlled substance, ketamine requires production and supply from a manufacturer possessing MHRA manufacturing authorization which ensures the production of good manufacturing practice (GMP) quality ketamine. Additionally, like in the US, because IV ketamine has not yet been granted marketing authorization for the psychotherapy indication in the UK, it must be regarded as an unlicensed medicine that is being used off label without its authorized indications for anesthesia and/or chronic pain. The GMC code of good practice allows a physician to prescribe an unlicensed medicine under his own responsibility.
Specifically, in the UK, our operation process are as follows:
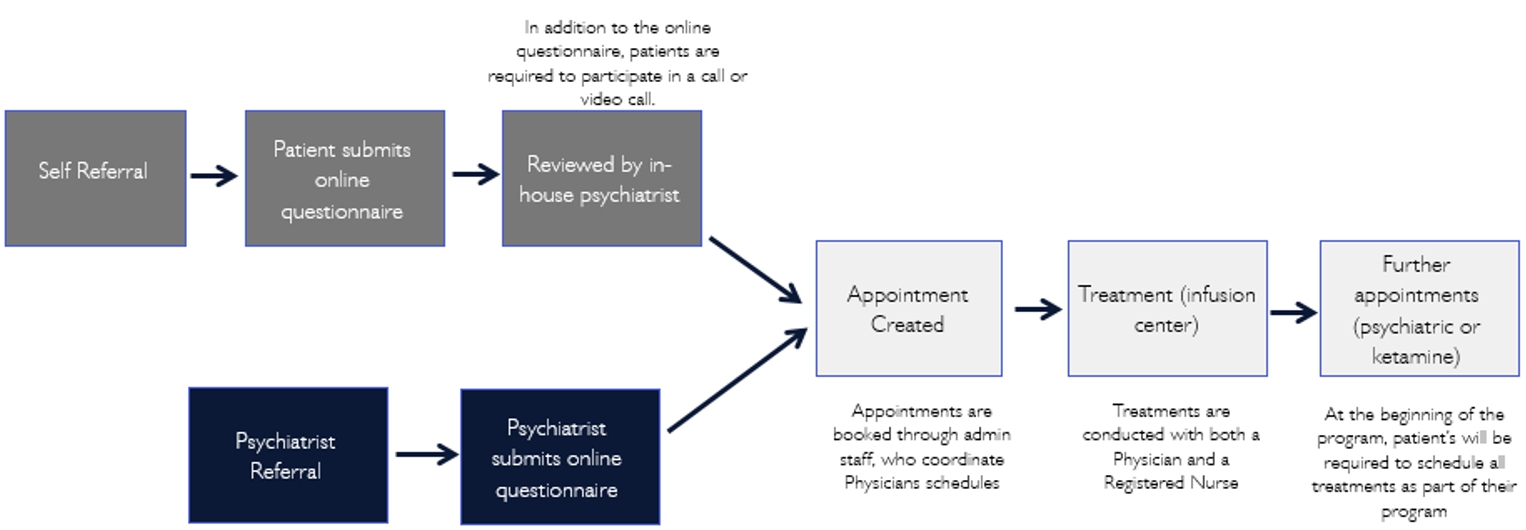
3
United States (including New York and California). In the United States, Pasithea has partnered with The IV Doc. The IV Doc itself and through clinical affiliates has treated over 50,000 patients over the past seven years. During that period, The IV Doc has established relationships with over 800 clinicians. Through these efforts, The IV Doc has developed a national reputation for the provision of in-home infusion services, testing, and outpatient medical care. Pasithea’s operations in New York and Los Angeles can be efficiently expanded to other locations utilizing The IV Doc patient service delivery model, including The IV Doc software and technology and clinical services management resources.
In the United States, the FDA, the Drug Enforcement Agency (DEA) and state agencies regulate the use, maintenance and distribution of ketamine. At the federal level, FDA has approved ketamine for use as an anesthetic but not for subanesthetic intravenous administration for psychotherapy. However, in general, physicians may prescribe FDA-approved drugs for conditions other than what the drugs have been explicitly approved for (off-label use). Once a drug such as ketamine is approved for any use, physicians may prescribe those drugs for off-label uses consistent with applicable state medical practice requirements (see below). Thus, no new or additional approvals are required from the FDA for the off-label use of ketamine for proper medical use like in this instance. The DEA, under the federal Controlled Substance Act, oversees the maintenance and distribution of all controlled substances, including ketamine. Depending on the specific clinical protocols and standards established by the independent professional services company and the contracted or employed physicians prescribing and administering ketamine, the entity and/or the contracted or employed physicians will be required to comply with all DEA requirements.
In New York and California (the “Initial States”), Pasithea is in the process of establishing management agreements with independent professional services companies that are in the process of formation that will be organized and established under the laws of the Initial States, including all laws related to the corporate practice of medicine, fee-splitting, licensure, and fraud and abuse laws. The independent professional services companies, through their physicians and nurses, will perform the clinical operations related to the business model. Individual clinicians, including psychiatrists, anesthesiologists, and nurses, all licensed and qualified to provide the clinical services required, will contract with the independent professional services companies to provide the services (see below). The independent professional services companies will be owned by a separate shareholder or shareholders from Pasithea. Through its management agreement, Pasithea, in conjunction with The IV Doc, will provide all of the non-clinical management services necessary for the professional services companies to operate, including administrative services, information technology services and marketing services, online advertising, and other channels. Pasithea has entered into a subcontract agreement with The IV Doc to provide for the subcontracting of certain administrative, information technology, and billing services provided by The IV Doc to us.
In New York, licensed New York psychiatrists will perform the initial diagnostic services to patients. Thereafter, these patients will be evaluated and, where appropriate, administered IV ketamine by licensed anesthesiologists. For further discussion, you should read the section titled “Business - Clinical Services” beginning on page 60.
In California, licensed psychiatrists will perform the initial diagnostic services to patients. Thereafter, these patients will be evaluated and, where appropriate, administered IV ketamine by licensed anesthesiologists. For further discussion, you should read the section titled “Business - Clinical Services” beginning on page 60.
4
The process of ketamine infusion treatment entails first conducting a psychiatric assessment of the patient to determine the appropriateness of the treatment, then administering infusion treatment, and finally conducting psychiatric follow-ups. Specifically, in the United States, our operation process are as follows (in the United States, Pasithea will only provide business support services to other entities providing the relevant medical services):
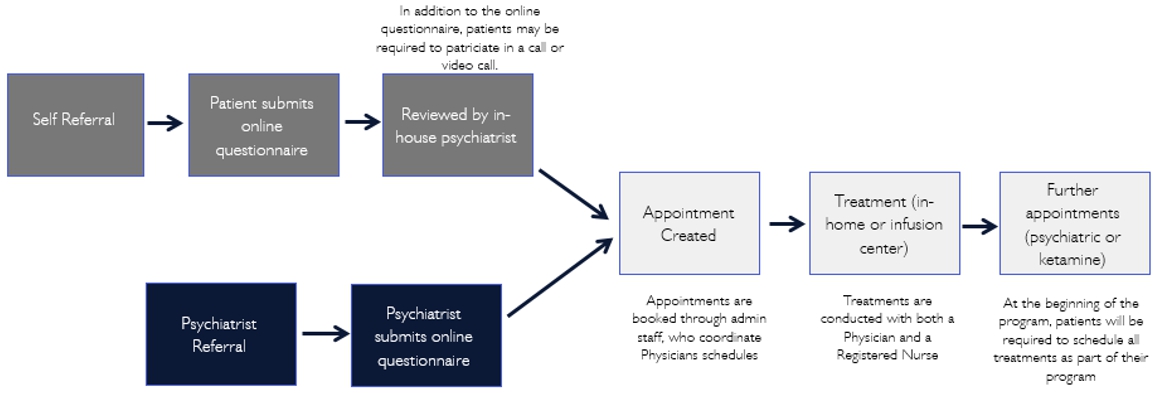
Our Team
We are founded and led by a best-in-class management team:
| ● | Professor Lawrence Steinman, Executive Chairman and Co-Founder. Professor Steinman has served on our board of directors since August 2020. Prior to joining Pasithea, he served on the Board of Directors of Centocor from 1989 to 1998, the Board of Directors of Neurocine Biosciences from 1997 to 2005, the Board of Directors of Atreca from 2010 to 2019, the Board of Directors of BioAtla from 2016 to the present, and the Board of Directors of Tolerion from 2013 to the present. He is currently the George A. Zimmermann Endowed Chair in the Neurology Department at Stanford University and previously served as the Chair of the Interdepartmental Program in Immunology at Stanford University Medical School from 2003 to 2011. He is a member of the National Academy of Medicine and the National Academy of Sciences. He also founded the Steinman Laboratory at Stanford University, which is dedicated to understanding the pathogenesis of autoimmune diseases, particularly multiple sclerosis and neuromyelitis optica. He received the Frederic Sasse Award from the Free University of Berlin in 1994, the Sen. Jacob Javits Award from the U.S. Congress in 1988 and 2002, the John Dystel Prize in 2004 from the National MS Society in the U.S., the Charcot Prize for Lifetime Achievement in Multiple Sclerosis Research in 2011 from the International Federation of MS Societies and the Anthony Cerami Award in Translational Medicine by the Feinstein Institute of Molecular Medicine in 2015. He also received an honorary Ph.D. at the Hasselt University in 2008. He received his BA (physics) from Dartmouth College in 1968 and his MD from Harvard University in 1973. He also completed a fellowship in chemical immunology at the Weizmann Institute (1974 – 1977) and was an intern and resident at Stanford University Medical School. | |
| ● | Dr. Tiago Reis Marques, Chief Executive Officer and Director. Dr. Marques has served on our board of directors and as Chief Executive Officer since August 2020. He is a senior clinical fellow at Imperial College London and a lecturer at the Institute of Psychiatry, Psychology & Neuroscience (IoPPN), King’s College London. IoPPN is ranked second in the world for psychology and psychiatry by US News and Best Global Universities, and is home to one of the world’s largest centers for neuroscience research. Dr. Marques is also a psychiatrist at Maudsley Hospital. His research focuses on topics including the mechanism of action of psychiatric medication and novel treatment targets. During his career, he has obtained multiple awards for his research. Dr. Marques is an author or co-author of more than 100 scientific publications in peer-reviewed journals in psychiatry and neuroscience, has co-authored international treatment guidelines and written book chapters, including in the leading book in the field, “Neurobiology of Mental Illness.” | |
| ● | Stanley M. Gloss, Chief Financial Officer. Mr. Gloss has served as our Chief Financial Officer since April 2021. He has been self-employed for the past year doing financial consulting in the areas of accounting and financial reporting. From 2017 to 2020, Mr. Gloss was Controller at Ace Universe, establishing and maintaining the budgets and financial reporting systems and sourcing and maintaining the company insurance. From 2009 to 2016, Mr. Gloss was Controller and Vice President of Finance of Wizard World Inc., where he established and maintained the budgets and financial reporting systems, sourced and maintained the company contracts and insurance, and coordinated public filings. He received his Bachelor of Science in Accounting from Fairfield University. |
5
| ● | Dr. Yassine Bendiabdallah, Chief Operating Officer, Head of UK Clinics and Director. Dr. Bendiabdallah has served on our board of directors and as Chief Operating Officer since March 2021. He also co-founded Pasithea Therapeutics Corp. and is currently Head of UK Clinics. Dr. Bendiabdallah is an expert in functional medicine and bio-identical hormone therapy. He completed a Masters in Pharmacy at King’s College London in 2006. He was then awarded a PhD scholarship within Cancer Research UK group at University Colleges London which was completed with honours in 2010. He then went on to work for a number of pharmaceutical companies and held research position at University College London. He has been involved in several startups including HelloDr (HelloDr Ltd, Proximal Health Ltd) an online tech in healthcare, Androgenix Pharmaceuticals Ltd, and Purecare Ltd (Zen Healthcare) which he is the co-founder and current managing director. Zen Healthcare now comprises several clinics and pharmacies in the UK. He holds a number of scientific publications in peer-reviewed literature the anticancer research industry. Dr. Bendiabdallah has also attended and presented at several seminars and conferences globally. His current clinical expertise includes age reversal therapies, functional approaches to medicines and intravenous micronutrient therapies. |
| ● | Simon Dumesnil, Director. Mr. Dumesnil has served on our board of directors since April 2021. He is currently a Managing Partner and Director of Dunraven Capital Partners Limited, an investment management advisory company incorporated in the UK whose investments are predominately in Eastern European corporate distressed credits and structured products. From 2013 to 2018, Mr. Dumesnil was Managing Director and Head of Structured Financing Group Americas of UBS Securities LLC, where he was responsible for the structured financing trading book in the USA and LATAM and managed a book of financing positions across fixed income products (corporate syndicated and middle-market loans, corporate bonds, real estate loans, CMBS/RMBS/CLO/ABS, LATAM Sovereign). From 2010 to 2013, he was Managing Director and Co-Head Private-Side Structuring Group EMEA of UBS AG., where he was responsible for arranging structured solution transactions and acquisitions for FIG and Special Situation Group (SSG) and also co-headed the illiquid financing business. From 2009 to 2010, Mr. Dumesnil was the Chief Investment Officer Bluestone Capital Management and responsible for investments in distressed assets across Europe. From 2008 to 2009, Mr. Dumesnil was Director of Lehman Brother Holding Inc. and responsible for restructuring and unwinding Lehman Brothers Special Financing Inc. derivative book post-bankruptcy. From 2003 to 2008, Mr. Dumesnil was Director of Lehman Brothers International (Europe). Throughout his career at Dunraven Capital Management, UBS Securities, UBS AG, Bluestone Capital Management and Lehman Brothers, Mr. Dumesnil advised and underwritten corporate risk related to companies across industries or jurisdictions. He has an in-depth knowledge on corporate restructuring and capital structure optimization for companies across their business life cycle. His experience as Chief Investment Officer during the launch and growth phases of a financial services and technology company represents valuable insights for our Company. Mr. Dumesnil attended Cass Business School, where he received his Master of Science in Banking and International Finance and École des Hautes-Études-Commerciales HEC, where he received his Bachelor in Business and Administration, Finance. |
Other Partnerships
In addition to our clinic partnerships described above, we anticipate partnering both with contract research organizations and educational institutions to help develop our product candidates and, eventually, to support our clinical trials.
Manufacturing
We anticipate devoting significant resources to process development and manufacturing to optimize process robustness and success rates in developing potential product candidates with financially viable per-unit manufacturing costs and enable us to quickly achieve regional and global scale production upon regulatory approval for our future product candidates.
Financial Overview
We have experienced losses since inception and, at December 31, 2020, had an accumulated deficit of approximately $40,984. We expect to incur additional losses in the future and expect cumulative losses to increase. Since May 2020, we have received approximately $1.47 million in equity financing in connection with which we issued 8,307,327 shares of Common Stock to approximately 46 accredited investors through a series of financings conducted pursuant to the Rule 506(b) Regulation D “safe harbor” for the private offering exemption of Section 4(a)(2) of the Securities Act completed in January 2021.
6
Summary of Risk Factors
Our business and operations are subject to a number of risks, which you should be aware of prior to making a decision to invest in our Common Stock. These risks are discussed more fully in the “Risk Factors” section of this prospectus immediately following this prospectus summary. Below is a summary of these risks.
Risks Relating to our Business
| ● | We have a limited operating history and have no products or services approved for commercial sale, which may make it difficult for you to evaluate our current business and predict our future success and viability. | |
| ● | If the potential of our future product candidates to treat diseases is not realized, the value of our technology and our development programs could be significantly reduced. | |
| ● | Our future product candidates may cause side effects that could delay or prevent their regulatory approval or have other significant adverse implications on our business, financial condition and results of operations. | |
| ● | If we are not able to recruit and retain qualified management and scientific personnel, we may fail in developing our technologies and product candidates. | |
| ● | Our future product candidates will represent new classes of therapy that the marketplace may not understand or accept. | |
| ● | If we fail to comply with any of the privacy and data security requirements of being a HIPAA “business associate”, we could be subject to significant liability, which can adversely affect our business. | |
| ● | We may eventually compete for product sales with other companies, many of which will have greater resources or capabilities than we have, or may succeed in developing better products or in developing products more quickly than we do, and we may not compete successfully with them. | |
| ● | We have ongoing challenges with respect to our liquidity and access to capital. | |
| ● | We have a history of losses and may not be able to achieve profitability going forward. | |
| ● | Public health threats including those related to the novel strain of coronavirus, SARS-CoV-2 (which causes the disease now called COVID-19), could have an adverse effect on our operations. | |
| ● | If we are unable to effectively adopt to changes in the health care industry, our revenue, profitability or liquidity could be adversely affected. | |
| ● | If our labor costs continue to rise, we may experience disruptions in our business operations and increases in operating expenses, among other things, which could have a material adverse effect on our business, results of operations, financial condition and cash flows. | |
| ● | Our internal computer systems, or those of our future CROs, manufacturers, contractors, consultants, or collaborators, may fail or suffer security or data privacy breaches or other unauthorized access to, use of, or destruction of our proprietary or confidential data, employee data, or personal data, which could result in additional costs, loss of revenue, significant liabilities, harm to our brand and material disruption of our operations. | |
| ● | A variety of risks associated with marketing our future product candidates internationally could materially adversely affect our business. | |
| ● | We may face limitations on ownership of controlled substances licenses. | |
| ● | We plan to operate in a highly regulated sector and may not always succeed in complying fully with applicable regulatory requirements in all jurisdictions where we carry on business. | |
| ● | We may not be able to successfully engage physicians and other healthcare professionals in need of our services. |
Risks Relating to Intellectual Property
| ● | If our trade secret and patent position does not adequately protect our future product candidates and uses, others could compete against us more directly, which could harm our business and have a material adverse effect on our business, financial condition and results of operations. | |
| ● | If we are unable to protect the confidentiality of our proprietary information, trade secrets, and know-how, our competitive position could be impaired and our business, financial condition, results of operations, and prospects could be adversely affected. | |
| ● | Third-party claims of intellectual property infringement may prevent or delay our product development efforts. | |
| ● | We may become involved in lawsuits to protect or enforce our future patents or the patents of our collaborators or licensors, which could be expensive and time consuming. | |
| ● | Intellectual property litigation may lead to unfavorable publicity that harms our reputation and causes the market price of our common shares to decline. | |
| ● | Patent reform legislation could increase the uncertainties and costs surrounding the prosecution of any future patents applications and the enforcement or defense of any future patents. | |
| ● | Changes in U.S. patent law, or laws in other countries, could diminish the value of patents in general, thereby impairing our ability to protect our future product candidates. | |
| ● | Patent terms may be inadequate to protect our competitive position on our future product candidates for an adequate amount of time. | |
| ● | If we or our licensors do not obtain patent term extension for our future product candidates and/or methods of their use, our business may be materially harmed. |
Risks Related to Regulatory Approval and Other Governmental Regulations
● |
If we are not able to successfully develop and commercialize our product candidates and obtain the necessary regulatory approvals, we may not generate sufficient revenues to continue our business operations. |
|
| ● | Any product candidates we may develop in the future may be subject to controlled substance laws and regulations in the territories where the product may be marketed and failure to comply with these laws and regulations, or the cost of compliance, may adversely affect the results of our business operations. | |
● |
The potential reclassification of certain substances in the United States could create additional regulatory burdens on our operations and negatively affect our results of operations. |
|
● |
We cannot market and sell our future product candidates in the U.S. or in other countries if we fail to obtain the necessary regulatory approvals. |
|
●
|
Even if our future product candidates receive regulatory approval in the U.S., we may never receive approval or commercialize our future product candidates outside of the U.S. |
|
●
|
Final marketing approval of our future product candidates by regulatory authorities for commercial use may be delayed, limited, or denied, any of which could adversely affect our ability to generate operating revenues. |
|
● |
We may not be able to secure and maintain research institutions to conduct our clinical trials. |
|
● |
Producing and marketing an approved drug or other medical product is subject to significant and costly post-approval regulation. |
|
| ● | We face exposure to fraudulent or illegal activity. | |
● |
If current or future laws or regulations force us to restructure our arrangements with physician practices, we may incur additional costs, lose contracts and suffer a reduction in net revenue under existing contracts. |
7
Risks Related to Our Dependence on Third Parties
| ● | We have not yet entered into agreements with independent professional services companies or other potential counterparties relating to our ketamine infusion business in the United States. | |
| ● | We may rely on third parties to provide us with supplies to produce our future product candidates. Any problems experienced by these third parties could have a material negative effect on our business. | |
| ● | Use of third-party manufacturers may increase the risk that we will not have adequate quantities of our future product candidates. | |
| ● | If we decide to use third-party manufacturers in the future, they will likely be dependent upon their own third-party suppliers, making us vulnerable to supply shortages and price fluctuations, which could harm our business. | |
| ● | We are subject to a multitude of manufacturing risks, any of which could substantially increase our costs and limit supply of our future product candidates. | |
| ● | We will depend on third-party distributors in the future to market and sell our future product candidates which will subject us to a number of risks. | |
| ● | The successful commercialization of our future product candidates will depend on obtaining reimbursement from government and third-party payors. | |
| ● | We may enter into arrangements with third-party collaborators to help us develop our product candidates and commercialize our products, and our ability to commercialize such products may be impaired or delayed if collaborations are unsuccessful. | |
| ● | If we engage in future acquisitions or strategic partnerships, this may increase our capital requirements, dilute our stockholders, cause us to incur debt or assume contingent liabilities, and subject us to other risks. | |
| ● | A shortage of qualified registered nursing staff and other caregivers could adversely affect our partners’ ability to attract, train and retain qualified personnel and could increase operating costs. | |
| ● | We anticipate generating revenue and profit margin under contracts with medical professional entities, and will face risks related to entering and retaining such contracts. | |
| ● | Any non-compete agreements and other restrictive covenants involving physicians may not be enforceable. | |
| ● | Failure of our affiliated physicians and other medical practitioners to comply with laws and regulations could result in suspension or revocation of our affiliated physicians’ licenses and termination of our service agreements. |
Risks Related to the Discovery, Development and Commercialization of Our Future Product Candidates
| ● | Interim, “topline” and preliminary data from our future clinical trials that we announce or publish may change as more data become available and are subject to audit and verification procedures that could result in material changes in the final data. | |
| ● | We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on other product candidates or indications that may be more profitable or for which there is a greater likelihood of success. | |
| ● | The FDA and other comparable foreign regulatory authorities may not accept data from trials conducted in locations outside of their jurisdiction. | |
| ● | Obtaining and maintaining regulatory approval of a product in one jurisdiction does not mean that we will be successful in obtaining or maintaining regulatory approval in other jurisdictions. | |
| ● | The FDA and other regulatory agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses. | |
| ● | We may attempt to secure approval from the FDA or comparable foreign regulatory authorities through an expedited review program, and if we are unable to do so, then we could face increased expense to obtain, and delays in the receipt of, necessary marketing approvals. | |
| ● | We may face difficulties from changes to current regulations and future legislation, both in the U.S. as well as in other foreign jurisdictions where we may be operating. | |
| ● | Our relationships with healthcare professionals, clinical investigators, clinical research organizations (CROs) and third-party payors in connection with our current and future business activities may be subject to federal and state healthcare fraud and abuse laws, false claims laws, transparency laws, government price reporting, and health information privacy and security laws, which could expose us to criminal sanctions, civil penalties, contractual damages, exclusion from governmental healthcare programs, reputational harm, administrative burdens and diminished profits and future earnings. | |
| ● | Inadequate funding for the FDA and other government agencies, future government shutdown, furlough of government employees, or public health emergencies could hinder their ability to hire and retain key personnel, prevent new products and services from being reviewed or approved in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of our business may rely, which could negatively impact our business. | |
| ● | If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on our business, financial condition, and results of operations. | |
| ● | Our research and development activities could be affected or delayed as a result of possible restrictions on animal testing. | |
| ● | Our business activities may be subject to the U.S. Foreign Corrupt Practices Act (FCPA) and similar anti-bribery and anti-corruption laws of other countries in which we operate, as well as U.S. and certain foreign export controls, trade sanctions, and import laws and regulations. Compliance with these legal requirements could limit our ability to compete in foreign markets and subject us to liability if we violate them. |
Risks Related to Employee Matters, Managing Our Growth and Other Risks Related to Our Business
| ● | We have never commercialized a product candidate before and may lack the necessary expertise, personnel and resources to successfully commercialize any products on our own or together with suitable collaborators. | |
| ● | In order to successfully implement our plans and strategies, we will need to grow our organization, and we may experience difficulties in managing this growth. | |
| ● | We cannot assure you that our plans to raise capital will be successful. |
8
Risks Related to this Offering and Ownership of Our Common Stock
| ● | There has been no prior public market for our Common Stock. We do not know whether an active, liquid and orderly trading market will develop for our Common Stock or what the market price of our Common Stock will be and as a result it may be difficult for you to sell your shares of our Common Stock. | |
| ● | The price of our stock may be volatile, and you could lose all or part of your investment. | |
| ● | Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our future product candidates on unfavorable terms to us. | |
| ● | If securities or industry analysts do not publish research or reports, or if they publish adverse or misleading research or reports, regarding us, our business or our market, our stock price and trading volume could decline. | |
| ● | Our quarterly operating results may fluctuate significantly or may fall below the expectations of investors or securities analysts, each of which may cause our stock price to fluctuate or decline. | |
| ● | If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, stockholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our Common Stock. | |
| ● | If you purchase shares of our Common Stock in our initial public offering, you will experience substantial and immediate dilution. | |
| ● | Sales of a substantial number of shares of our Common Stock in the public market could cause our stock price to fall. | |
| ● | We are an “emerging growth company,” and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our Common Stock less attractive to investors. | |
| ● | The requirements of being a public company may strain our resources, result in more litigation and divert management’s attention. | |
| ● | We may be subject to securities litigation, which is expensive and could divert management attention. | |
| ● | We do not currently intend to pay dividends on our Common Stock and, consequently, your ability to achieve a return on your investment will depend on appreciation of the value of our Common Stock. | |
| ● | Provisions in our certificate of incorporation and bylaws and Delaware law might discourage, delay or prevent a change in control of our company or changes in our management and, therefore, depress the market price of our Common Stock. | |
| ● | There is no guarantee that our Common Stock will be listed on Nasdaq. |
Implications of Being an Emerging Growth Company and a Smaller Reporting Company
We qualify as an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, as amended, or JOBS Act. As an “emerging growth company” we may take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
| ● | the option to present only two years of audited financial statements and only two years of related “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in this prospectus; | |
| ● | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended, or the Sarbanes-Oxley Act; | |
| ● | not being required to comply with any requirements that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (i.e., an auditor discussion and analysis); | |
| ● | reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and | |
| ● | exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
We may take advantage of these provisions until the last day of our fiscal year following the fifth anniversary of the completion of this offering. However, if any of the following events occur prior to the end of such five-year period, (i) our annual gross revenue exceeds $1.07 billion, (ii) we issue more than $1.0 billion of non-convertible debt in any three-year period, or (iii) we become a “large accelerated filer,” (as defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)), we will cease to be an emerging growth company prior to the end of such five-year period. We will be deemed to be a “large accelerated filer” at such time that we (a) have an aggregate worldwide market value of common equity securities held by non-affiliates of $700 million or more as of the last business day of our most recently completed second fiscal quarter, (b) have been required to file annual and quarterly reports under the Exchange Act for a period of at least 12 months and (c) have filed at least one annual report pursuant to the Exchange Act. Even after we no longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company,” which would allow us to take advantage of many of the same exemptions from disclosure requirements including reduced disclosure obligations regarding executive compensation in this prospectus and our periodic reports and proxy statements.
We have elected to take advantage of certain of the reduced disclosure obligations in the registration statement of which this prospectus is a part and may elect to take advantage of other reduced reporting requirements in future filings. As a result, the information that we provide to our stockholders may be different than you might receive from other public reporting companies in which you hold equity interests.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. We have elected to take advantage of this extended transition period.
Corporate Information
We were formed as a Delaware corporation in May 2020. Our principal executive offices are located at 1111 Lincoln Road, Suite 500, Miami Beach, FL 33139 and our telephone number is (702) 514-4174. Our website address is www.pasithea.com. The information contained in, or accessible through, our website does not constitute a part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
9
The Offering
| Common stock offered by us | shares. | |
| Option to purchase additional shares | We have granted the underwriters an option for a period of 45 days to purchase up to additional shares of Common Stock. | |
| Common stock to be outstanding after this offering | shares (or shares if the underwriters exercise their option to purchase additional shares in full). | |
| Use of proceeds | We estimate that the net proceeds from this offering will be approximately $ million (or approximately $ million if the underwriters exercise their option to purchase additional shares in full), based on an assumed initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. We intend to use the net proceeds of this offering for research and development (including clinical trials and product development), to develop our U.S. clinic and UK clinic business, and for working capital and other general corporate purposes. For a more complete description of our intended use of the proceeds from this offering, see “Use of Proceeds.” | |
| Underwriters’ warrants | Upon the closing of this offering, we have agreed to issue to Kingswood Capital Markets, division of Benchmark Investments, Inc., as representative of the underwriters, warrants that will be exercisable for the period commencing six months from the effective date of this offering and expiring five years from the effective date of the offering, entitling the representative to purchase 5% of the number of shares of Common Stock sold in this offering. The registration statement of which this prospectus is a part also covers the underwriters’ warrants and the Common Stock issuable upon the exercise thereof. For additional information regarding our arrangement with the underwriters, please see “Underwriting.” | |
| Lock-up agreements | We and our executive officers, directors and certain of our stockholders have agreed with the underwriters not to sell, transfer or dispose of any shares or similar securities for certain periods of time after the date of this prospectus. For additional information regarding our arrangement with the underwriters, please see “Underwriting.” | |
| Risk factors | You should read the section titled “Risk Factors” beginning on page 15 and the other information included in this prospectus for a discussion of factors you should consider carefully before deciding to invest in our Common Stock. | |
| Proposed Nasdaq Capital Market symbol | “KTTA.” | |
The number of shares of our Common Stock to be outstanding after this offering is based on 8,307,327 shares of our Common Stock outstanding as of April 13, 2021 and excludes:
| ● | shares of Common Stock issuable upon exercise of warrants to be issued to the representative of the underwriters as part of this offering at an exercise price of $ (assuming an initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus)). |
Except as otherwise indicated herein, all information in this prospectus assumes or gives effect to:
| ● | Effective April 8, 2021, we amended our certificate of incorporation to effect a 1-for-20 reverse stock split of our outstanding shares of Common Stock. No fractional shares will be issued as a result of the reverse stock split. Any fractional shares resulting from the reverse stock split shall be paid in cash. The reverse stock split does not otherwise affect any of the rights currently accruing to holders of our Common Stock. All share information presented in this prospectus has been retroactively adjusted to reflect the reduced number of shares outstanding. | |
● |
no exercise by the underwriters of their option to purchase additional shares of our Common Stock in this offering. |
10
SUMMARY FINANCIAL DATA
The following tables set forth our summary financial data for the periods indicated. We have derived the statements of operations data for the period from May 12, 2020 (inception) to December 31, 2020, and the balance sheet data as of December 31, 2020, from our audited financial statements included elsewhere in this prospectus. We have prepared the unaudited financial statements on the same basis as the audited financial statements and have included all adjustments, consisting only of normal recurring adjustments that, in our opinion, are necessary to state fairly the financial information set forth in those statements. Our historical results are not necessarily indicative of the results that should be expected for any future period. You should read the following summary financial data together with the more detailed information contained in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the related notes included elsewhere in this prospectus.
| Year
Ended December 31, 2020 | ||||
| Operating expenses: | ||||
| Selling, general and administrative | $ | 40,984 | ||
| Loss from operations | (40,984 | ) | ||
| Loss before income taxes | (40,984 | ) | ||
| Benefit from (provision for) income taxes | - | |||
| Net income (loss) | (40,984 | ) | ||
| Weighted-average common shares outstanding, basic and diluted | 7,364,166 | |||
| Basic and diluted net loss per common share | (0.00 | ) | ||
| As
of December 31, 2020 | ||||||||
| Actual | As Adjusted(1)(2) | |||||||
| Balance Sheet Data: | ||||||||
| Cash and cash equivalents | $ | 243,650 | ||||||
| Working capital(3) | $ | 241,355 | ||||||
| Total assets | $ | 247,958 | ||||||
| Total liabilities | $ | 6,603 | ||||||
| Accumulated deficit | $ | (40,984 | ) | |||||
| Total equity | $ | 241,355 | ||||||
| (1) | The as adjusted balance sheet data gives effect to the issuance and sale of shares of Common Stock in this offering at an assumed initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
| (2) | Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) as adjusted cash and cash equivalents, working capital, total assets, and total equity by $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase (decrease) of 100,000 shares in the number of shares offered by us at the assumed initial public offering price, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us would increase (decrease) as adjusted cash and cash equivalents, working capital, total assets, and total equity by $ million. The as adjusted information discussed above is illustrative only and will be adjusted based on the actual initial public offering price and other terms of our initial public offering determined at pricing. |
| (3) | We define working capital as current assets less current liabilities. |
11
You should carefully consider the risks and uncertainties described below and the other information in this prospectus, including our financial statements and related notes appearing elsewhere in this prospectus and in the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” before deciding whether to invest in our Common Stock. Our business, financial condition, results of operations or prospects could be materially and adversely affected if any of these risks occurs, and as a result, the market price of our Common Stock could decline and you could lose all or part of your investment. This prospectus also contains forward-looking statements that involve risks and uncertainties. See “Special Note Regarding Forward-Looking Statements.” Our actual results could differ materially and adversely from those anticipated in these forward-looking statements as a result of certain factors, including those set forth below. For a summary of these risk factors, please see “Summary of Risk Factors” in the section titled “Prospectus Summary” beginning on page 1 of this prospectus.
Risks Relating to our Business
We have a limited operating history and have no products or services approved for commercial sale, which may make it difficult for you to evaluate our current business and predict our future success and viability.
We are a clinical stage biotechnology company with a limited operating history upon which you can evaluate our business and prospects. We have no products or services approved for commercial sale and have not generated any material revenue from product sales. To date, we have devoted substantially all of our resources and efforts to organizing and staffing our company, business planning, building and equipping our research and development laboratories, building and equipping our manufacturing suites, raising capital, acquiring raw materials for manufacturing, product candidate development and manufacturing, and securing related intellectual property rights. We have not yet demonstrated our ability to obtain marketing approvals, manufacture a commercial-scale product or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. As a result, it may be more difficult for you to accurately predict our future success or viability than it could be if we had a longer operating history.
In addition, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors and risks frequently experienced by clinical stage biotechnology companies in rapidly evolving fields, including, but not limited to, changes in FDA or foreign body regulatory oversight of such products. We also may need to transition from a company with a research focus to a company capable of supporting commercial activities. Such a transition may involve substantial additional capital requirements in order to launch and market a product, changes in the use of proceeds, and significant adjustment to personnel, compared to a clinical-stage development company. If we do not adequately address these risks and difficulties or successfully make such a transition, our business will suffer.
If the potential of our future product candidates to treat diseases is not realized, the value of our technology and our development programs could be significantly reduced.
Our team is currently exploring the potential of our future product candidates to treat psychiatric and neurological disorders. We have not yet proven in clinical trials that our future product candidates will be a safe and effective treatment for any disease or condition. Our future product candidates are susceptible to various risks, including undesirable and unintended side effects, unintended immune system responses, inadequate therapeutic efficacy, or other characteristics that may prevent or limit their marketing approval or commercial use. We have not yet completed all of the testing necessary to allow us to make a determination that serious unintended consequences will not occur. If the potential of our future product candidates to treat disease is not realized, the value of our technology and our development programs could be significantly reduced.
Our future product candidates may cause undesirable side effects that could delay or prevent their regulatory approval or commercialization or have other significant adverse implications on our business, financial condition and results of operations.
Undesirable side effects observed in clinical trials or in supportive preclinical studies with our future product candidates could interrupt, delay or halt their development and could result in the denial of regulatory approval by the FDA or comparable foreign authorities for any or all targeted indications or adversely affect the marketability of any such product candidates that receive regulatory approval. In turn, this could eliminate or limit our ability to commercialize our future product candidates.
12
Our future product candidates may exhibit adverse effects in preclinical toxicology studies and adverse interactions with other drugs. There are also risks associated with additional requirements the FDA or comparable foreign authorities may impose for marketing approval with regard to a particular disease.
Our future product candidates may require a risk management program that could include patient and healthcare provider education, usage guidelines, appropriate promotional activities, a post-marketing observational study, and ongoing safety and reporting mechanisms, among other requirements. Prescribing could be limited to physician specialists or physicians trained in the use of the drug, or could be limited to a more restricted patient population. Any risk management program required for approval of our future product candidates could potentially have an adverse effect on our business, financial condition and results of operations.
Undesirable side effects involving our future product candidates may have other significant adverse implications on our business, financial condition and results of operations. For example:
| ● | we may be unable to obtain additional financing on acceptable terms, if at all; |
| ● | our collaborators may terminate any development agreements covering these product candidates; |
| ● | if any development agreements are terminated, we may determine not to further develop the affected product candidates due to resource constraints and may not be able to establish additional collaborations for their further development on acceptable terms, if at all; |
| ● | if we were to later continue the development of these product candidates and receive regulatory approval, earlier findings may significantly limit their marketability and thus significantly lower our potential future revenues from their commercialization; |
| ● | we may be subject to product liability or stockholder litigation; and |
| ● | we may be unable to attract and retain key employees. |
In addition, if any of our future product candidates receive marketing approval and we or others later identify undesirable side effects caused by the product:
| ● | regulatory authorities may withdraw their approval of the product, or we or our partners may decide to cease marketing and sale of the product voluntarily; |
| ● | we may be required to change the way the product is administered, conduct additional clinical trials or preclinical studies regarding the product, change the labeling of the product, or change the product’s manufacturing facilities; and |
| ● | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of the affected product and could substantially increase the costs and expenses of commercializing the product, which in turn could delay or prevent us from generating significant revenues from the sale of the product.
13
If we are not able to recruit and retain qualified management and scientific personnel, we may fail in developing our technologies and our future product candidates.
Our future success depends to a significant extent on the skills, experience, and efforts of the principal members of our scientific and management personnel. These members include Professor Lawrence Steinman, Dr. Tiago Reis Marques and our staff of scientific consultants. The loss of any or all of these individuals could harm our business and might significantly delay or prevent the achievement of research, development or business objectives. Competition for regulatory, clinical manufacturing and management personnel in the pharmaceutical industry is intense. We may be unable to recruit or retain personnel with sufficient management skills or attract or integrate other qualified management and scientific personnel in the future.
Our future product candidates will represent new classes of therapy that the marketplace may not understand or accept.
Even if we successfully develop and obtain regulatory approval for our product candidates, the market may not understand or accept them. We anticipate developing product candidates that represent novel treatment approaches and will compete with a number of more conventional products and therapies manufactured and marketed by others, including major pharmaceutical companies. The degree of market acceptance of any of our developed and potential products will depend on a number of factors, including:
| ● | the clinical safety and effectiveness of our products and their perceived advantage over alternative treatment methods; |
| ● | our ability to demonstrate that our products can have a clinically significant effect in the treatment of depression and mental illness for which we may seek marketing approval; |
| ● | our ability to develop drugs that show efficacy for the treatment of psychiatric and neurological disorders; |
| ● | our ability to supply a sufficient amount of our products to meet regular and repeated demand in order to develop a core group of medical professionals familiar with and committed to the use of our products; and |
| ● | the cost of our products and the reimbursement policies of government and third-party payors. |
If the health care community does not accept our future product candidates or future approved products for any of the foregoing reasons, or for any other reason, it could affect our sales or have a material adverse effect on our business, financial condition, results of operations, and prospects.
We expect to function as a HIPAA “business associate” as defined under HIPAA and, as such, we expect to be subject to strict privacy and data security requirements. If we fail to comply with any of these requirements, we could be subject to significant liability, all of which can adversely affect our business.
The Health Insurance Portability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”), and their respective implementing regulations (“HIPAA”), imposes specified requirements relating to the privacy, security and transmission of individually identifiable health information. Among other things, HITECH makes HIPAA’s security standards directly applicable to “business associates.” We expect to function as a business associate of HIPAA covered entities and service providers, and in that context we are regulated as a business associate for the purposes of HIPAA. If we are unable to comply with our obligations as a HIPAA business associate, we could face substantial civil and even criminal liability. Modifying the already stringent penalty structure that was present under HIPAA prior to HITECH, HITECH created four new tiers of civil monetary penalties and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions. In addition, many state laws govern the privacy and security of health information in certain circumstances, many of which differ from HIPAA and each other in significant ways and may not have the same effect.
14
The HIPAA covered entities and service providers to which we provide services require us to enter into HIPAA-compliant business associate agreements with them. These agreements impose stringent data security obligations on us. If we are unable to meet the requirements of any of these business associate agreements, we could face contractual liability under the applicable business associate agreement as well as possible civil and criminal liability under HIPAA, all of which can have an adverse impact on our business and generate negative publicity.
We may eventually compete for product sales with other companies, many of which will have greater resources or capabilities than we have, or may succeed in developing better products or in developing products more quickly than we do, and we may not compete successfully with them. Other companies and research institutions may obtain licenses or authorizations for drugs or for drugs with similar pharmacologies before we do which may affect our commercialization.
We compete or may eventually compete with other companies and organizations that are marketing or developing therapies for our targeted disease indications, based on traditional pharmaceutical, medical device, or other technologies. In addition, we have other potential competitors developing a variety of therapeutics, and in some cases, there may be tens or hundreds of companies seeking to commercialize therapeutics. The pharmaceutical market for the treatment of major depressive disorder includes selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors and atypical antipsychotics. A number of these marketed antidepressants will be generic, and would be key competitors to our ketamine drug candidate. These products include Janssen Pharmaceuticals, Inc.’s Spravato (esketamine), Forest Laboratory’s Lexapro/Cipralex (escitalopram) and Viibryd (vilazodone), Pfizer, Inc.’s Zoloft (sertraline), Effexor (venlafaxine) and Pristiq (desvenlafaxine), GlaxoSmithKline plc’s Paxil/Seroxat (paroxetine), Eli Lilly and Company’s Prozac (fluoxetine) and Cymbalta (duloxetine), AstraZeneca plc’s Seroquel (quetiapine) and Bristol-Myers Squibb Company’s Abilify (aripiprazole), among others.
We anticipate that competition in our industry will increase. In addition, the health care industry is characterized by rapid technological change, resulting in new product introductions and other technological advancements. Our competitors may develop and market products that render future product candidates, or any products manufactured or marketed by us, non-competitive or otherwise obsolete.
We have ongoing challenges with respect to our liquidity and access to capital.
As we advance the development of our programs, we expect to continue to incur significant expenses and operating losses, for which we do not have offsetting revenue. We expect that our sales, research and development and general and administrative costs will increase in connection with conducting preclinical studies and clinical trials for our future programs and product candidates, contracting with contract research organizations (CROs) to support preclinical studies and clinical trials, establishing, and providing general and administrative support for our operations. As a result, we will need additional capital to fund our operations, which we may obtain from additional equity or debt financings, collaborations, licensing arrangements, or other sources.
Since May 2020, we have received approximately $1.47 million in equity financing. As of December 31, 2020, we had $243,650 in cash and cash equivalents and working capital of approximately $241,355. There are no assurances that we will be able to continue to finance operations through these means, and our inability to generate sufficient revenue in the near term may have an adverse impact on our business, operations and prospects.
We have a history of losses and may not be able to achieve profitability going forward.
We have experienced losses since inception and, at December 31, 2020, had an accumulated deficit of approximately $40,984. We expect to incur additional losses in the future and expect the cumulative losses to increase. There is no assurance that operating expenses will remain at current levels, nor that any potential grant revenue will fund our clinical programs. In such event, we will not have sufficient cash flow to meet our obligations or make progress in our clinical programs, and will need to raise additional capital to provide sufficient funding.
15
Public health threats, including those related to the novel strain of coronavirus, SARS-CoV-2 (which causes the disease now called COVID-19), could have an adverse effect on our operations.
Public health threats could adversely affect our planned research and development activities. In particular, SARS-CoV-2, which causes the disease now called COVID-19, was first reported to have surfaced in Wuhan, China in December 2019, and has since spread globally, including to every state in the United States. On January 31, 2020, the Secretary of Health and Human Services (HHS) issued a Public Health Emergency determination in response to the spread of COVID-19. Numerous state and local jurisdictions have imposed, and others in the future may impose, “shelter-in-place” orders, quarantines, executive orders and similar government orders and restrictions for their residents to control the spread of COVID-19. Starting in mid-March 2020, the governor of New York issued “shelter-in-place” or “stay at home” orders restricting non-essential activities, travel and business operations for an indefinite period of time, subject to certain exceptions for necessary activities. Similar orders and restrictions have been imposed in California and Massachusetts. Even after the “shelter-in-place” orders, quarantines, executive orders and similar government orders and restrictions for their residents to control the spread of COVID-19 are lifted, we may continue to experience disruptions to our business. The outbreak of COVID-19 has severely impacted global economic activity and caused significant volatility and negative pressure in financial markets. The global impact of the outbreak has been rapidly evolving and many countries, including the United States, have reacted by instituting quarantines, mandating business and school closures and restricting travel. As a result, the COVID-19 pandemic is negatively impacting almost every industry directly or indirectly.
We cannot presently predict the scope and severity of any potential business shutdowns or disruptions, but if we or any of the third parties with whom we engage, including the suppliers, clinical trial sites, regulators and other third parties with whom we conduct business, were to experience shutdowns or other business disruptions, our ability to conduct our business in the manner and on the timelines presently planned could be materially and negatively impacted.
The spread of an infectious disease, including COVID-19, may also result in the inability of our suppliers to deliver components or raw materials on a timely basis. Such events may result in a period of business and manufacturing disruption, and in reduced operations, any of which could materially affect our business, financial condition and results of operations. The extent to which the coronavirus impacts our business will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of COVID-19 and the actions to contain the coronavirus or treat its impact, among others.
If we are unable to effectively adapt to changes in the health care industry, our revenue, profitability or liquidity could be adversely affected.
The health care industry continues to experience significant change driven by efforts to reduce costs and improve standards of care. In addition to reduction in Medicare, Medicaid and third-party reimbursement, these efforts include potential national health care reform, increased and restrictive pharmacy benefit management and horizontal and vertical consolidation within the health care industry. The results of these efforts may put additional downward pressure on pricing for our products and services, which may adversely affect our revenue, profitability or liquidity. Our inability to react effectively to these and other changes in the health care industry could adversely affect our business.
If our labor costs continue to rise, including due to shortages, changes in certification requirements and/or higher than normal turnover rates in skilled clinical personnel; or currently pending or future governmental laws, rules, regulations or initiatives impose additional requirements or limitations on our operations or profitability; or, if we are unable to attract and retain key leadership talent, we may experience disruptions in our business operations and increases in operating expenses, among other things, which could have a material adverse effect on our business, results of operations, financial condition and cash flows.
We face increasing labor costs generally, and in particular, we continue to face increased labor costs and difficulties in hiring nurses due to a nationwide shortage of skilled clinical personnel that has been exacerbated by the ongoing COVID-19 pandemic. We have incurred and expect to continue to incur increased labor costs and experience staffing challenges related to COVID-19 while the pandemic persists, the extent of which will depend on the severity and duration of the pandemic, among other things. We compete for nurses with hospitals and other healthcare providers. This nursing shortage may limit our ability to expand our operations. Furthermore, changes in certification requirements can impact our ability to maintain sufficient staff levels, including to the extent our teammates are not able to meet new requirements, among other things. In addition, if we experience a higher than normal turnover rate for our skilled clinical personnel, our operations and treatment growth may be negatively impacted, which could adversely affect our business, results of operations, financial condition and cash flows. We also face competition in attracting and retaining talent for key leadership positions. If we are unable to attract and retain qualified individuals, we may experience disruptions in our business operations, including, without limitation, our ability to achieve strategic goals, which could have a material adverse effect on our business, results of operations, financial condition and cash flows.
16
Our internal computer systems, or those of any of our future CROs, manufacturers, other contractors, consultants, or collaborators, may fail or suffer security or data privacy breaches or other unauthorized or improper access to, use of, or destruction of our proprietary or confidential data, employee data, or personal data, which could result in additional costs, loss of revenue, significant liabilities, harm to our brand and material disruption of our operations.
Despite the implementation of security measures, our internal computer systems and those of our current and any future CROs and other contractors, consultants, collaborators and third-party service providers, are vulnerable to damage from computer viruses, cybersecurity threats, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failure. If such an event were to occur and cause interruptions in our operations or result in the unauthorized acquisition of or access to personally identifiable information or individually identifiable health information (violating certain privacy laws such as the Health Insurance Portability and Accountability Act of 1996, Health Information Technology for Economic and Clinical Health Act and the EU Regulation 2016/679, the General Data Protection Regulation (GDPR)), it could result in a material disruption of our drug discovery and development programs and our business operations, whether due to a loss of our trade secrets or other similar disruptions. Some of the federal, state and foreign government requirements include obligations of companies to notify individuals of security breaches involving particular personally identifiable information, which could result from breaches experienced by us or by our vendors, contractors, or organizations with which we have formed strategic relationships. Notifications and follow-up actions related to a security breach could impact our reputation, cause us to incur significant costs, including legal expenses and remediation costs. For example, the loss of clinical trial data from future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the lost data. We also rely on third parties to manufacture our future product candidates, and similar events relating to their computer systems could also have a material adverse effect on our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data, or inappropriate disclosure of confidential or proprietary information, we could be exposed to litigation and governmental investigations, the further development and commercialization of our future product candidates could be delayed, and we could be subject to significant fines or penalties for any noncompliance with certain state, federal and/or international privacy and security laws.
We currently do not have insurance policies to compensate us for the potential losses arising from any such disruption, failure or security breach, and we may not be able to obtain insurance policies on favorable terms. In addition, such insurance may not be available to us in the future on economically reasonable terms, or at all. Further, our insurance may not cover all claims made against us and could have high deductibles in any event, and defending a suit, regardless of its merit, could be costly and divert management attention.
A variety of risks associated with marketing our future product candidates internationally could materially adversely affect our business.
We plan to seek regulatory approval of our future product candidates outside of the United States, and, accordingly, we expect that we will be subject to additional risks related to operating in foreign countries if we obtain the necessary approvals, including:
| ● | differing regulatory requirements and reimbursement regimes in foreign countries; | |
| ● | unexpected changes in tariffs, trade barriers, price and exchange controls and other regulatory requirements; | |
| ● | economic weakness, including inflation, or political instability in particular foreign economies and markets; | |
| ● | compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; | |
| ● | foreign taxes, including withholding of payroll taxes; | |
| ● | foreign currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident to doing business in another country; | |
| ● | difficulties staffing and managing foreign operations; | |
| ● | workforce uncertainty in countries where labor unrest is more common than in the United States; |
17
| ● | potential liability under the FCPA or comparable foreign regulations; | |
| ● | challenges enforcing our contractual and intellectual property rights, especially in those foreign countries that do not respect and protect intellectual property rights to the same extent as the United States; | |
| ● | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and | |
| ● | business interruptions resulting from geo-political actions, including war and terrorism. |
These and other risks associated with our international operations may materially adversely affect our ability to attain or maintain profitable operations.
We may face limitations on ownership of controlled substances licenses.
In certain states, the controlled substances laws and regulations limit not only the number of licenses issued, but also the number of licenses that one person or entity may own. Such limitations on the ownership of additional licenses within certain states may limit our ability to expand in such states.
We plan to operate in a highly regulated sector and may not always succeed in complying fully with applicable regulatory requirements in all jurisdictions where we carry on business.
Our business and activities are heavily regulated in all jurisdictions where we plan to carry on business. Our operations will be subject to various laws, regulations and guidelines by state and local governmental authorities relating to the manufacture, marketing, management, transportation, storage, and also including laws and regulations relating to health and safety, insurance coverage, the conduct of operations and the protection of the environment. Laws and regulations, applied generally, grant government agencies and self-regulatory bodies broad administrative discretion over our activities, including the power to limit or restrict business activities as well as impose additional disclosure requirements on our products and services. Achievement of our business objectives is contingent, in part, upon compliance with regulatory requirements enacted by these governmental authorities and obtaining all necessary regulatory approvals for the manufacture, production, storage, transportation, sale, import and export, as applicable, of our products. The industry is still a new industry at the state and local level. The effect of relevant governmental authorities’ administration, application and enforcement of their respective regulatory regimes and delays in obtaining, or failure to obtain, applicable regulatory approvals which may be required may significantly delay or impact the development of markets, products and sales initiatives and could have a material adverse effect on our business, prospects, revenue, results of operation and financial condition.
While we endeavor to comply with all relevant laws, regulations and guidelines and, to our knowledge, we are in compliance or are in the process of being assessed for compliance with all such laws, regulations and guidelines, any failure to comply with the regulatory requirements applicable to our operations may lead to possible sanctions including the revocation or imposition of additional conditions on licenses to operate our business; the suspension or expulsion from a particular market or jurisdiction or of our key personnel; the imposition of additional or more stringent inspection, testing and reporting requirements; and the imposition of fines and censures. In addition, changes in regulations, more vigorous enforcement thereof or other unanticipated events could require extensive changes to our operations, increase compliance costs or give rise to material liabilities and/or revocation of our licenses and other permits, which could have a material adverse effect on our business, results of operations and financial condition. Furthermore, governmental authorities may change their administration, application or enforcement procedures at any time, which may adversely impact our ongoing costs relating to regulatory compliance.
We may not be able to successfully engage physicians and other healthcare professionals in need of our services.
Our ability to engage physicians and other healthcare professionals will affect our performance. Our support services related to the infusion of ketamine are furnished to physicians with a greater degree of specialized skills, training and experience than in other areas of practice. This decreases the number of healthcare professionals who may be recipients of our services. Moreover, we compete with other entities to furnish business support services to physician practices. Our future success depends in part on our ability to engage physicians and other healthcare professionals to maintain and expand our operations.
18
Risks Relating to Intellectual Property
If our trade secret and patent position does not adequately protect our future product candidates and uses, others could compete against us more directly, which could harm our business and have a material adverse effect on our business, financial condition and results of operations.
Our success depends, in large part, on our ability to obtain and maintain intellectual property protection for our future product candidates. The patent position of biotechnology companies is generally highly uncertain, involves complex legal and factual questions, and continues to be the subject of much litigation. Our trade secrets will remain valid and enforceable without regard to limitations such as term restrictions that are imposed on patents. Our trade secrets and know-how are the subject of various license agreements and confidentiality agreements as further discussed below.
The claims of existing U.S. and foreign patent applications and patents, and those patents that may issue in the future, or those to be licensed to us, that are owned by the Company or under an obligation of assignment to the Company, may not confer on us significant commercial protection against competing products. Furthermore, to the extent that the Company owns or is assigned or licenses patent rights covering its business, third parties may challenge or design around those patent rights, such as by asserting that the patents are invalid or arguing that the patent claims should be narrowly construed, and thereby avoid infringement actions. The laws of foreign countries may not protect our intellectual property rights to the same extent as do the laws of the United States.
Because of the extensive time required for development, testing, and regulatory review of a potential product, it is possible that, before any of our products can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantages of the patent. To the extent our future product candidates based on that technology are not commercialized ahead of this patent expiration, to the extent we have no other patent protection on such products, or to the extent that regulatory or patent extensions are not granted, those products might not have the robust protection we currently expect to enjoy. The background technologies used in the development of our future product candidates are known in the scientific community, and it may be possible to duplicate the methods we use to create our future product candidates, which makes us vulnerable to competition, without the ability to exclude others from potentially commercializing a similar product.
If we are unable to protect the confidentiality of our proprietary information, trade secrets, and know-how, our competitive position could be impaired and our business, financial condition, results of operations, and prospects could be adversely affected.
As disclosed above, some aspects of our technology, especially regarding manufacturing processes, are unpatented and maintained by us as trade secrets. In an effort to protect these trade secrets, we require our employees, consultants, collaborators, and advisors to execute confidential disclosure agreements before the commencement of their relationships with us. These agreements require that all confidential information developed by the individual or made known to the individual by us during the course of the individual’s relationship with us be kept confidential and not disclosed to third parties. These agreements, however, may not provide us with adequate protection against improper use or disclosure of confidential information, and these agreements may be breached. A breach of confidentiality could affect our competitive position. In addition, in some situations, these agreements may conflict with, or be subject to, the rights of third parties with whom our employees, consultants, collaborators, or advisors have previous employment or consulting relationships. Also, others may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets.
Adequate remedies may not exist in the event of unauthorized use or disclosure of our confidential information. The disclosure of our trade secrets could impair our competitive position and could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Third-party claims of intellectual property infringement may prevent or delay our product development efforts.
Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. There is a substantial amount of litigation involving patents and other intellectual property rights in the biotechnology and pharmaceutical industries. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we will develop our product candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our future product candidates, methods of making product candidates, and methods of using product candidates may give rise to claims of infringement of the patent rights of others.
19
Third parties may assert that we infringe their patents or are otherwise employing their proprietary technology without authorization and may sue us. Generally, conducting clinical trials and other acts relating to FDA approval are not considered acts of infringement in the United States.
Additionally, there may be third-party patents of which we are currently unaware with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our future product candidates. Because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that our future product candidates may infringe. Some of those patent applications may not yet be available for public inspection. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. If any third-party patents were held by a court of competent jurisdiction to cover the manufacturing process of our future product candidates, constructs or molecules used in or formed during the manufacturing process, or any final product itself, the holders of any such patents may be able to block our ability to commercialize the product candidate unless we obtained a license under the applicable patents, or until such patents expire or they are finally determined to be held not infringed, unpatentable, invalid or unenforceable. Similarly, if any third-party patent were held by a court of competent jurisdiction to cover aspects of our formulations, processes for manufacture or methods of use, including combination therapy or patient selection methods, the holders of any such patent may be able to block our ability to develop and commercialize the product candidate unless we obtained a license or until such patent expires or is finally determined to be held not infringed, unpatentable, invalid or unenforceable. In either case, such a license may not be available on commercially reasonable terms or at all. If we are unable to obtain a necessary license to a third-party patent on commercially reasonable terms, or at all, our ability to commercialize our future product candidates may be impaired or delayed, which could in turn significantly harm our business.
Parties making claims against us may seek and obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize our future product candidates. They might seek an exclusion order from the International Trade Commission to prevent import of our future product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business and may impact our reputation. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay royalties or redesign our infringing products, which may be impossible or require substantial time and monetary expenditure. We cannot predict whether any such license would be available at all or whether it would be available on commercially reasonable terms. Furthermore, even in the absence of litigation, we may need to obtain licenses from third parties to advance our research or allow commercialization of our future product candidates. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, we would be unable to further develop and commercialize our future product candidates, which could harm our business significantly.
We may become involved in lawsuits to protect or enforce our future patents or the patents of our collaborators or licensors, which could be expensive and time consuming.
Litigation may be necessary to enforce future patents licensed to us, to protect trade secrets or know-how, or to determine the scope and validity of the proprietary rights. Litigation, opposition, or other patent office proceedings could result in substantial additional costs and diversion of management focus. If we are ultimately unable to protect our technology, trade secrets, or know-how, we may be unable to operate profitably. Competitors may infringe any future patents or the patents of our collaborators or licensors. As a result, we may be required to file infringement claims to protect our proprietary rights, which can be expensive and time-consuming, particularly for a company of our size. In addition, in an infringement proceeding, a court may decide that a patent of ours is invalid or is unenforceable, or may refuse to enjoin the other party from using the technology at issue. An adverse determination of any litigation or defense proceedings could put any future patents at risk of being invalidated or interpreted narrowly. Litigation or other patent office proceedings may fail and, even if successful, may result in substantial costs and distraction to our management. We may not be able, alone or with our collaborators and licensors, to prevent misappropriation of our proprietary rights, particularly in countries where the laws may not protect such rights as fully as in the United States.
Furthermore, though we would seek protective orders where appropriate, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, during the course of this kind of litigation, there could be public announcements of the results of hearings, motions, or other interim proceedings or developments. If investors perceive these results to be negative, the market price for our Common Stock could be significantly harmed.
20
The biotechnology industry, including our fields of therapeutic interest, is highly competitive and subject to significant and rapid technological change. Accordingly, our success may depend, in part, on our ability to respond quickly to such change through the development and introduction of new products. Our ability to compete successfully against currently existing and future alternatives to our future product candidates and systems and competitors who compete directly with us in the biopharmaceutical industry may depend, in part, on our ability to attract and retain skilled scientific and research personnel, develop technologically superior products, develop competitively priced products, obtain patent or other required regulatory approvals for our products, and be early entrants to the market and manufacture, market, and sell our products, independently or through collaborations. If a third party were to commercialize a competitive product, there is no assurance that we would have a basis for initiating patent infringement proceedings or that, if initiated, we would prevail in such proceedings.
If our future product candidates are approved by the FDA, then potential competitors who seek to introduce generic versions of our product candidates may seek to take advantage of the abbreviated approval pathway for products shown to be similar to or interchangeable with our product candidates. The Biologics Price Competition and Innovation Act of 2009 might permit these potential competitors to enter the market using a shorter and less costly development program for a biosimilar product that competes with our future products.
Intellectual property litigation may lead to unfavorable publicity that harms our reputation and causes the market price of our common shares to decline.
During the course of any intellectual property litigation, there could be public announcements of the initiation of the litigation as well as results of hearings, rulings on motions, and other interim proceedings in the litigation. If securities analysts or investors regard these announcements as negative, the perceived value of our existing products, programs or intellectual property could be diminished. Accordingly, the market price of shares of our Common Stock may decline. Such announcements could also harm our reputation or the market for our future products, which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Patent reform legislation could increase the uncertainties and costs surrounding the prosecution of any future patents applications and the enforcement or defense of any future patents.
In September 2011, the Leahy-Smith America Invents Act, or Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications are prosecuted and may also affect patent litigation. In particular, under the Leahy-Smith Act, the United States transitioned in March 2013 to a “first inventor to file” system in which, assuming that other requirements of patentability are met, the first inventor to file a patent application will be entitled to the patent regardless of whether a third party was first to invent the claimed invention. A third party that files a patent application in the United States Patent and Trademark Office (USPTO) after March 2013 but before us could therefore be awarded a patent covering an invention of that we also made even if we had made the invention before the invention was made independently by such third party. This will require us to be cognizant going forward of the time from invention to filing of a patent application. Furthermore, our ability to obtain and maintain valid and enforceable patents depends on whether the differences between our technology and the prior art allow our technology to be patentable over the prior art. Since patent applications in the United States and most other countries are confidential for a period of time after filing or until issuance, we cannot be certain that we will be the first to either (1) file any patent application related to our future product candidates or (2) invent any of the inventions claimed in any future patent applications.
The Leahy-Smith Act also includes a number of significant changes that affect the way patent applications will be prosecuted and also may affect patent litigation. These include allowing third-party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent by USPTO administered post-grant proceedings, including post-grant review, inter partes review, and derivation proceedings. An adverse determination in any such submission or proceeding could reduce the scope or enforceability of, or invalidate, any future patents rights, which could adversely affect our competitive position.
Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in United States federal courts necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a patent claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate any future patents claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action. Thus, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our or licensors’ patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
21
Changes in U.S. patent law, or laws in other countries, could diminish the value of patents in general, thereby impairing our ability to protect our future product candidates.
As is the case with other biopharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involve a high degree of technological and legal complexity. Therefore, obtaining and enforcing biopharmaceutical patents is costly, time-consuming and inherently uncertain. Changes in either the patent laws or in the interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property and may increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. We cannot predict the breadth of claims that may be allowed or enforced in any future patents or in third-party patents. In addition, Congress or other foreign legislative bodies may pass patent reform legislation that is unfavorable to us.
For example, the U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our or our licensors’ ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the U.S. federal courts, the USPTO, or similar authorities in foreign jurisdictions, the laws and regulations governing patents could change in unpredictable ways that would weaken our or our licensors’ ability to obtain new patents or to enforce our existing patents and patents we might obtain in the future.
Patent terms may be inadequate to protect our competitive position on our future product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the term of a patent, and the protection it affords, are limited. Even if patents directed to our product candidates are obtained, once the patent term has expired, we may be open to competition from competitive products. Given the amount of time required for the development, testing and regulatory review of future product candidates, patents directed to our future product candidates might expire before or shortly after such candidates are commercialized.
If we or our licensors do not obtain patent term extension for our future product candidates and/or methods of their use, our business may be materially harmed.
Depending upon the timing, duration and specifics of FDA marketing approval of our future product candidates and their methods of use, one or more of our U.S. patents may be eligible for limited patent term restoration under the Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Amendments, or the Biologics Price Competition and Innovation Act of 2009. These laws permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. A maximum of one patent may be extended per FDA-approved product as compensation for the patent term lost during the FDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only those claims covering such approved drug product, a method for using it or a method for manufacturing it may be extended.
Patent term extension may also be available in certain foreign countries upon regulatory approval of our future product candidates. However, we or our licensors may not be granted an extension because of, for example, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents or otherwise failing to satisfy applicable requirements. Patent term extension may also not be granted because the product candidates and/or methods of use are determined not to be the first permitted marketing or use of those drug candidates in the jurisdiction in question, or patent term extension may not be granted because the product candidates and/or methods of use are determined not to constitute an “active ingredient” or use of an “active ingredient” that is eligible for patent term extension. Moreover, if patent term extension is granted then the additional time period or the scope of patent protection afforded could be less than we request. If we or our licensors are unable to obtain patent term extension or restoration or the term of any such extension is less than we request, our competitors may obtain approval of competing products following any future patent expiration, and our revenue could be reduced, possibly materially. Further, if this occurs, our competitors may take advantage of our investment in development and trials by referencing our clinical and preclinical data and launch their product earlier than might otherwise be the case.
22
Risks Related to Regulatory Approval and Other Government Regulations
If we are not able to successfully develop and commercialize our product candidates and obtain the necessary regulatory approvals, we may not generate sufficient revenues to continue our business operations.
To generate sales revenue from our future product candidates, we must conduct extensive preclinical studies and clinical trials to demonstrate that our future product candidates are safe and effective and we must obtain required regulatory approvals. Our early stage product candidates may fail to perform as we expect. Moreover, our future product candidates in later stages of development may fail to show the required safety and effectiveness for approval despite having progressed successfully through preclinical or initial clinical testing. We may need to devote significant additional research and development, financial resources, and personnel to develop commercially viable products. If our future product candidates do not prove to be safe and efficacious in clinical trials, we will not obtain the required regulatory approvals. If we fail to obtain such approvals, we may not generate sufficient revenues to continue our business operations.
Even if we obtain regulatory approval of a product, that approval may be subject to limitations on the indicated uses for which it may be marketed. Even after granting regulatory approval, the FDA and regulatory agencies in other countries continue to review and inspect marketed products, manufacturers, and manufacturing facilities, which may create additional regulatory burdens. Later discovery of previously unknown problems with a product, manufacturer, or facility may result in restrictions on the product or manufacturer, including a withdrawal of the product from the market or a withdrawal of the approved application by the FDA. Furthermore, FDA may require post-approval studies or other commitments from us, and failure to comply with or meet those commitments could result in withdrawal of the approved application by FDA. Regulatory agencies may also establish additional regulations, policies, or guidance that could prevent or delay regulatory approval of our future product candidates.
Any product candidates we may develop in the future may be subject to controlled substance laws and regulations in the territories where the product may be marketed, such as the U.S. and the U.K., and failure to comply with these laws and regulations, or the cost of compliance, may adversely affect the results of our business operations, both during clinical development and post approval, and our financial condition. In addition, during the review process of our future product candidates, and prior to approval, the FDA and/or other regulatory bodies may require additional data, including with respect to whether our future product candidates have abuse potential, which may delay approval and any potential rescheduling process.
In the U.S., certain substances are classified by the Drug Enforcement Administration (the “DEA”) as “Controlled Substances” or scheduled substances, under the Comprehensive Drug Abuse Prevention and Control Act of 1970, also known as the Controlled Substances Act, or CSA, specifically as a Schedule I substance. The DEA regulates chemical compounds as Schedule I, II, III, IV or V substances. Schedule I substances by definition have a high potential for abuse, have no currently “accepted medical use” in the United States, lack accepted safety for use under medical supervision, and may not be prescribed, marketed or sold in the United States. Pharmaceutical products approved for use in the United States may be listed as Schedule II, III, IV or V, with Schedule II substances considered to present the highest potential for abuse or dependence and Schedule V substances the lowest relative risk of abuse among such substances. Schedule I and II drugs are subject to the strictest controls under the CSA, including manufacturing and procurement quotas, security requirements and criteria for importation. In addition, dispensing of Schedule II drugs is further restricted. For example, they may not be refilled without a new prescription and may have a black box warning. Commercial marketing in the United States will also require scheduling-related legislative or administrative action.
Scheduling determinations by the DEA are dependent on FDA approval of a substance or a specific formulation of a substance. Therefore, even though our future product candidates may contain a Schedule I controlled substance, products approved by the FDA for medical use in the United States that contain Schedule I substances should be placed in Schedules II-V, since approval by the FDA satisfies the “accepted medical use” requirement. If and when our future product candidates receive FDA approval, we anticipate that the DEA will make a scheduling determination and place it in a schedule other than Schedule I in order for it to be prescribed to patients in the United States. This scheduling determination will be dependent on FDA approval and the FDA’s recommendation as to the appropriate schedule. During the review process, and prior to approval, the FDA may determine that it requires additional data, either from non-clinical or clinical studies, including with respect to whether, or to what extent, the substance has abuse potential. This may introduce a delay into the approval and any potential rescheduling process. That delay would be dependent on the quantity of additional data required by the FDA. This scheduling determination will require DEA to conduct notice and comment rule making including issuing an interim final rule. Such action will be subject to public comment and requests for hearing which could affect the scheduling of these substances. There can be no assurance that the DEA will make a favorable scheduling decision. Even assuming categorization as a Schedule II or lower controlled substance (i.e., Schedule III, IV or V), at the federal level, such substances would also require scheduling determinations under state laws and regulations.
23
If approved by the FDA, and if the finished dosage form of our future product candidates is listed by the DEA as a Schedule II, III, or IV controlled substance, its manufacture, importation, exportation, domestic distribution, storage, sale and legitimate use will continue to be subject to a significant degree of regulation by the DEA. In addition, the scheduling process may take significantly longer than the 90-day deadline set forth in the CSA, thereby delaying the launch of our future product candidates in the United States. Furthermore, the FDA, DEA, or any foreign regulatory authority could require us to generate more clinical or other data than we currently anticipate to establish whether or to what extent the substance has an abuse potential, which could increase the cost and/or delay the launch of our future product candidates. In addition, therapeutic candidates containing controlled substances are subject to DEA regulations relating to manufacturing, storage, distribution and physician prescription procedures, including:
| ● | DEA registration and inspection of facilities. Facilities conducting research, manufacturing, distributing, importing or exporting, or dispensing controlled substances must be registered (licensed) to perform these activities and have the security, control, recordkeeping, reporting and inventory mechanisms required by the DEA to prevent drug loss and diversion. All these facilities must renew their registrations annually, except dispensing facilities, which must renew every three years. The DEA conducts periodic inspections of certain registered establishments that handle controlled substances. Obtaining and maintaining the necessary registrations may result in delay of the importation, manufacturing or distribution of our future product candidates. Furthermore, failure to maintain compliance with the CSA, particularly non-compliance resulting in loss or diversion, can result in regulatory action that could have a material adverse effect on our business, financial condition and results of operations. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to restrict, suspend or revoke those registrations. In certain circumstances, violations could lead to criminal proceedings. | |
| ● | State-controlled substances laws. Individual U.S. states have also established controlled substance laws and regulations. Though state-controlled substances laws often mirror federal law, because the states are separate jurisdictions, they may separately schedule our future product candidates. While some states automatically schedule a drug based on federal action, other states schedule drugs through rule making or a legislative action. State scheduling may delay commercial sale of any future product candidates for which we obtain federal regulatory approval and adverse scheduling could have a material adverse effect on the commercial attractiveness of such future product candidates. We or our partners must also obtain separate state registrations, permits or licenses in order to be able to obtain, handle, and distribute controlled substances for clinical trials or commercial sale, and failure to meet applicable regulatory requirements could lead to enforcement and sanctions by the states in addition to those from the DEA or otherwise arising under federal law. | |
| ● | Clinical trials. Because our future product candidates may contain certain controlled substances, to conduct clinical trials with our future product candidates in the United States prior to approval, each of our research sites must submit a research protocol to the DEA and obtain and maintain a DEA researcher registration that will allow those sites to handle and dispense the future product candidates and to obtain the future product candidates from our importer. If the DEA delays or denies the grant of a researcher registration to one or more research sites, the clinical trial could be significantly delayed, and we could lose clinical trial sites. The importer for the clinical trials must also obtain a Schedule I importer registration and an import permit for each import. We do not currently conduct any manufacturing or repackaging/relabeling of either the future product candidates or its active ingredients in the United States. |
| ● | Importation. If our future product candidates are approved and classified as a Schedule II, III or IV substance, an importer can import it for commercial purposes if it obtains an importer registration and files an application for an import permit for each import. The DEA provides annual assessments/estimates to the International Narcotics Control Board, which guides the DEA in the amounts of controlled substances that the DEA authorizes to be imported. The failure to identify an importer or obtain the necessary import authority, including specific quantities, could affect the availability of our future product candidates and have a material adverse effect on our business, results of operations and financial condition. In addition, an application for a Schedule II importer registration must be published in the Federal Register, and there is a waiting period for third-party comments to be submitted. It is always possible that adverse comments may delay the grant of an importer registration. If our future product candidates are approved and classified as a Schedule II controlled substance, federal law may prohibit the import of the substance for commercial purposes. If our future product candidates are listed as a Schedule II substance, we will not be allowed to import the drug for commercial purposes unless the DEA determines that domestic supplies are inadequate or there is inadequate domestic competition among domestic manufacturers for the substance as defined by the DEA. Moreover, Schedule I controlled substances have never been registered with the DEA for importation for commercial purposes, only for scientific and research needs. Therefore, if neither our future product candidates nor its drug substance could be imported, our future product candidates would have to be wholly manufactured in the United States, and we would need to secure a manufacturer that would be required to obtain and maintain a separate DEA registration for that activity. |
24
| ● | Manufacture in the United States. If, because of a Schedule II classification or voluntarily, we were to conduct manufacturing or repackaging/relabeling in the United States, our contract manufacturers would be subject to the DEA’s annual manufacturing and procurement quota requirements. Additionally, regardless of the scheduling of our future product candidates, the active ingredient in the final dosage form is likely to be a Schedule I controlled substance and would be subject to such quotas as this substance could remain listed on Schedule I. The annual quota allocated to us or our contract manufacturers for the active ingredient in our future product candidates may not be sufficient to complete clinical trials or meet commercial demand. Consequently, any delay or refusal by the DEA in establishing our, or our contract manufacturers’, procurement and/or production quota for controlled substances could delay or stop our clinical trials or future product candidates’ launches, which could have a material adverse effect on our business, financial position and results of operations. | |
| ● | Distribution in the United States. If our future product candidates are scheduled as Schedule II, III or IV, we would also need to identify wholesale distributors with the appropriate DEA registrations and authority to distribute the future product candidates and any future therapeutic candidates. These distributors would need to obtain Schedule II, III or IV distribution registrations. This limitation in the ability to distribute our future product candidates more broadly may limit commercial uptake and could negatively impact our prospects. The failure to obtain, or delay in obtaining, or the loss of any of those registrations could result in increased costs to us. If our future product candidates are Schedule II drugs, participants in our supply chain may have to maintain enhanced security with alarms and monitoring systems and they may be required to adhere to recordkeeping and inventory requirements. This may discourage some pharmacies from carrying the product. In addition, our future product candidates will likely be determined to have a high potential for abuse and therefore required to be administered at our trial sites, which could limit commercial update. Furthermore, state and federal enforcement actions, regulatory requirements, and legislation intended to reduce prescription drug abuse, such as the requirement that physicians consult a state prescription drug monitoring program, may make physicians less willing to prescribe, and pharmacies to dispense, Schedule II products. | |
Similarly, the MHRA considers that all Schedule 1 drugs under the UK’s Misuse of Drugs Regulations 2001 have no therapeutic benefit, and can only be imported, exported, produced, supplied and the like under a license issued by the UK Government’s Home Office. Our future product candidates and their compounds may never be rescheduled under the Misuse of Drugs Regulations 2001, or reclassified under the UK’s Misuse of Drugs Act 1971.
In the UK, entities in our supply chain, including third party collaborators in research or research sites, may be required to hold Home Office licenses and comply with necessary control measures. Import and export licenses may be required if sites are not located in the UK.
The potential reclassification of certain controlled substances in the United States could create additional regulatory burdens on our operations and negatively affect our results of operations.
If certain controlled substances that we may use in our future product candidates, other than the FDA-approved formulation, are rescheduled under the CSA as a Schedule II or lower controlled substance (i.e., Schedule III, IV or V), the ability to conduct research on these controlled substances would most likely be improved. However, rescheduling controlled substances may materially alter enforcement policies across many federal agencies, primarily the FDA and DEA. The FDA is responsible for ensuring public health and safety through regulation of food, drugs, supplements, and cosmetics, among other products, through its enforcement authority pursuant to the Federal Food, Drug, and Cosmetic Act, or the FDCA. The FDA’s responsibilities include regulating the ingredients as well as the marketing and labeling of drugs sold in interstate commerce. Because it is currently illegal under federal law to produce and sell certain Schedule I controlled substances, and because there are no federally recognized medical uses, the FDA has historically deferred enforcement related to these substances to the DEA. If these Schedule I substances were to be rescheduled to a federally controlled, yet legal, substance, the FDA would likely play a more active regulatory role. The DEA would continue to be active in regulating manufacturing, distribution and dispensing of such substances. The potential for multi-agency enforcement post-rescheduling could threaten or have a materially adverse effect on our business.
25
We cannot market and sell our future product candidates in the United States or in other countries if we fail to obtain the necessary regulatory approvals.
We cannot sell our future product candidates until regulatory agencies grant marketing approval. We have not previously submitted a New Drug Application, or NDA, to the FDA, or a Marketing Authorization Application, or MAA, to the EMA or the MHRA. Before obtaining regulatory approvals for the commercial sale of our product candidates or any future therapeutic candidates, we must demonstrate through lengthy, complex and expensive preclinical testing and clinical trials that our future product candidates are safe and effective for use in each target indication. Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process, and there is a high risk of failure and we may never succeed in developing marketable products.
The regulatory approval process of the FDA, the EMA, the MHRA, and comparable foreign authorities are lengthy, time-consuming, expensive, inherently unpredictable, and uncertain, and the legal requirements for obtaining approval may change. It is likely to take several years to obtain the required regulatory approvals for our future product candidates, or we may never gain the necessary approvals. Any difficulties that we encounter in obtaining regulatory approval may have a substantial adverse impact on our operations.
We may encounter delays or rejections if changes occur in regulatory agency regulations, policies or guidance during the period in which we develop a product candidates or during the period required for review of any application for regulatory agency approval. If we are not able to obtain regulatory approvals for use of our future product candidates under development, we will not be able to commercialize such products, and therefore may not be able to generate sufficient revenues to support our business.
Our future product candidates could fail to receive regulatory approval from the FDA, the EMA, the MHRA or comparable foreign regulatory authorities or be precluded from commercial marketing for many reasons, including the following:
| ● | the FDA, the EMA, the MHRA or comparable foreign regulatory authorities may disagree with, question or request changes in the design or implementation of our clinical trials; | |
| ● | the FDA, the EMA, the MHRA or comparable foreign regulatory authorities may determine that our product candidates are not safe and effective, only moderately effective, or have undesirable or unintended side effects, toxicities, or other characteristics that preclude our obtaining marketing approval or prevent or limit commercial use; | |
| ● | the results of clinical trials may not meet the level of statistical significance required by the FDA, the EMA, the MHRA or comparable foreign regulatory authorities for approval; |
| ● | we may be unable to demonstrate that our future product candidates or any future therapeutic candidate’s clinical and other benefits outweigh its safety risks; | |
| ● | the FDA, the EMA, the MHRA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials; | |
| ● | the data collected from clinical trials of our product candidates may not be sufficient to support the submission of an NDA or other submission, or to obtain regulatory approval in the United States or elsewhere; | |
| ● | the FDA, the EMA, the MHRA or comparable foreign regulatory authorities may find deficiencies with or fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; |
26
| ● | the approval policies or regulations of the FDA, the EMA, the MHRA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval; and | |
| ● | the potential risk of our novel therapy and delivery method, including the use of third-party clinical trial sites and therapists. | |
The FDA, the EMA, the MHRA and other comparable foreign authorities have substantial discretion in the approval process and determining when or whether regulatory approval will be obtained for our future product candidates or any future therapeutic candidates. Even if we believe the data collected from clinical trials of our future product candidates are promising, such data may not be sufficient to support approval by the FDA, the EMA, the MHRA or any other regulatory authority. If our future product candidates fail to obtain approval on the basis of any applicable condensed regulatory approval process, this will prevent such therapeutic candidate from obtaining approval on a shortened time frame, or at all, resulting in increased expenses which would materially harm our business.
In addition, even if we were to obtain approval, regulatory or pricing authorities may approve our future product candidates for fewer or more limited indications than we request, may not approve the price we intend to charge for our products or therapies, may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve a therapeutic candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that therapeutic candidate.
Even if our future product candidates receive regulatory approval in the U.S., we may never receive approval or commercialize our future product candidates outside of the U.S.
In order to market any products outside of the U.S., we must establish and comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy. Approval procedures vary among countries and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries might differ from that required to obtain FDA approval. The regulatory approval process in other countries may include all of the risks detailed above regarding FDA approval in the U.S. as well as other risks. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in others. Failure to obtain regulatory approval in other countries or any delay seeking or obtaining such approval would impair our ability to develop foreign markets for our future product candidates.
Our development costs will increase if we have material delays in our clinical trials, or if we are required to modify, suspend, terminate, or repeat a clinical trial. If we are unable to conduct our clinical trials properly and on schedule, marketing approval may be delayed or denied by the FDA.
Final marketing approval of our future product candidates by the FDA or other regulatory authorities for commercial use may be delayed, limited, or denied, any of which could adversely affect our ability to generate operating revenues.
Final marketing approval for our future product candidates may be delayed, limited, or denied if, among other factors:
| ● | we are unable to satisfy the significant clinical testing required to demonstrate safety and effectiveness of our future product candidates before marketing applications can be filed with the FDA; |
| ● | FDA does not agree with our interpretation of data obtained from preclinical and nonclinical animal testing and clinical trials, even though the data can be interpreted in different ways; |
| ● | we fail at any stage of the development and testing of our future product candidates, which may take years to complete; |
| ● | we receive negative or inconclusive results or reports of adverse side effects during a clinical trial; or |
| ● | the FDA requires us to expand the size and scope of the clinical trials. |
If marketing approval for our future product candidates is delayed, limited, or denied, our ability to market products, and our ability to generate product sales, could be adversely affected.
27
We may not be able to secure and maintain research institutions to conduct our clinical trials.
We rely on research institutions to conduct our clinical trials. Our reliance upon research institutions, including hospitals and clinics, provides us with less control over the timing and cost of clinical trials and the ability to recruit subjects. If we are unable to reach agreement with suitable research institutions on acceptable terms, or if any resulting agreement is terminated, we may be unable to quickly replace the research institution with another qualified institution on acceptable terms. Even if we do replace the institution, we may incur additional costs to conduct the trial at the new institution. We may not be able to secure and maintain suitable research institutions to conduct our clinical trials.
Producing and marketing an approved drug or other medical product is subject to significant and costly post-approval regulation.
Even if approved for commercial sale, we may be required to conduct Phase IV clinical trials or comply with other post-marketing requirements for our future product candidates. Even if we obtain approval of our future product candidates, we can only market the product for the approved indications. After granting marketing approval, the FDA and regulatory agencies in other countries continue to review and inspect marketed products, manufacturers, and manufacturing facilities, creating additional regulatory burdens. Later discovery of previously unknown problems with a product, manufacturer, or facility may result in restrictions on the product or manufacturer, including a withdrawal of the future product candidates from the market. Further, regulatory agencies may establish different or additional regulations that could impact the post-marketing status of our products.
We face exposure to fraudulent or illegal activity.
We face exposure to the risk that employees, independent contractors or consultants may engage in fraudulent or other illegal activities. Misconduct by these parties could be intentional, reckless and/or negligent conduct. There may be disclosure of unauthorized activities that violate government regulations, manufacturing standards, healthcare laws, abuse laws and other financial reporting laws. Further, it may not always be possible for us to identify and deter misconduct by our employees and other third parties, and the precautions we take to detect and prevent these activities may not always be effective. As a result, we could face potential penalties and litigation.
If current or future laws or regulations force us to restructure our arrangements with physician practices, we may incur additional costs, lose contracts and suffer a reduction in net revenue under existing contracts.
A number of laws bear on our relationships with our physicians. There is a risk that state authorities in some jurisdictions may find that our future contractual relationships with physician practices violate laws prohibiting the corporate practice of medicine and fee-splitting. These laws generally prohibit the practice of medicine by lay entities or persons and are intended to prevent unlicensed persons or entities from interfering with or inappropriately influencing the physician’s professional judgment. They may also prevent the sharing of professional services income with non-professional or business interests.
We intend to contract with physician practices organized as separate medical professional entities (e.g., professional medical corporations). Antitrust laws may deem each such physician/entity to be separate, both from us and from each other and, accordingly, each such physician/practice is subject to a wide range of laws that prohibit anti-competitive conduct between or among separate legal entities or individuals. A review or action by regulatory authorities or the courts could force us to terminate or modify our contractual relationships with affiliated medical groups or revise them in a manner that could be materially adverse to our business.
Various licensing laws, regulations and standards will apply to our affiliated physicians and our relationships with our affiliated physicians. Failure to comply with these laws and regulations could result in our services being found to be non-reimbursable or prior payments being subject to recoupment, and can give rise to civil or criminal penalties. While we have made reasonable efforts to ensure our affiliated physician practices and our relationships with our affiliated physician practices substantially comply with licensing laws and regulations and standards, we cannot assure you that agencies that administer these programs will not find that the affiliated practices or our relationships with our affiliated practices have failed to comply in some material respects.
28
Adverse judicial or administrative interpretations could result in a finding that we are not in compliance with one or more of these laws and rules that affect our relationships with our physicians.
These laws and rules, and their interpretations, may also change in the future. Any adverse interpretations or changes could force us to restructure our relationships with physicians or professional corporations, or to restructure our operations. This could cause our operating costs to increase significantly. A restructuring could also result in a loss of contracts or a reduction in revenue under existing contracts.
Risks Related to Our Dependence on Third Parties
We have not yet entered into agreements with independent professional services companies or other potential counterparties relating to our ketamine infusion business in the United States.
We have not yet entered into agreements with independent professional services companies or other potential counterparties relating to our ketamine infusion business in the United States and we may experience difficulty in executing such agreements on favorable terms, if at all.
We may rely on third parties to provide us with supplies to produce our future product candidates. Any problems experienced by these third parties could result in a delay or interruption in the supply of our future product candidates for our clinical trials and future approved products to our customers, which could have a material negative effect on our business.
We rely on third parties to provide us with supplies to produce our future product candidates. If the operations of these third parties are interrupted or if they are unable to meet our delivery requirements due to capacity limitations or other constraints, we may be limited in our ability to fulfill our supply and product candidate needs. Any prolonged disruption in the operations of third parties could have a significant negative impact on our ability to produce our future product candidates for pre-clinical and clinical trials or sell our future approved products, could harm our reputation and could cause us to seek other third-party contracts, thereby increasing our anticipated development and commercialization costs. In addition, if we are required to change third parties for any reason, we will be required to verify that the new third parties maintain facilities and procedures that comply with quality standards required by the FDA and with all applicable regulations and guidelines. The delays associated with the verification of a new third party could negatively affect our ability to develop product candidates or receive approval for any future product candidates in a timely manner.
We may become dependent upon third parties for services and raw materials needed for the manufacture of our future product candidates, and if these products are successfully commercialized, may become dependent upon third parties for product distribution. If any of these third parties fail or are unable to perform in a timely manner, our ability to manufacture and deliver could be compromised.
As we proceed with our clinical trial efforts, we must be able to demonstrate to the FDA that we can manufacture our future product candidates with consistent characteristics. While we plan to produce our future product candidates in our own facility, scaling up the manufacturing process would require us to develop a larger facility, which could require significant time and capital investments to conform to applicable manufacturing standards, or outsource manufacturing, which would cause us to be materially dependent on these suppliers for supply of GMP-grade components of consistent quality. Our ability to complete our future clinical trials may be negatively affected in the event that we are forced to seek and validate a replacement source for any of these critical components. If we are not able to obtain adequate supplies of these items of consistent quality from our third-party suppliers, it will also be more difficult to manufacture commercial quantities of our future product candidates that are approved for commercial sale.
In addition, if one or more of our future product candidates is approved for commercial sale, we intend to rely on third parties for their distribution. Proper shipping and distribution requires compliance with specific storage and shipment procedures (e.g., prevention of damage to shipping materials and prevention of temperature excursions during shipment). Failure to comply with such procedures will necessitate return and replacement, potentially resulting in additional cost and causing us to fail to meet supply requirements.
29
Use of third-party manufacturers may increase the risk that we will not have adequate quantities of our future product candidates.
We may use a third-party manufacturer to supply our future product candidates for clinical trials or other uses at some point. Reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured such components ourselves, including:
| ● | reliance on the third party for regulatory compliance and quality assurance; |
| ● | the possible breach of the manufacturing agreement by the third party; and |
| ● | the possible termination or nonrenewal of the agreement by the third party, based on its own business priorities, at a time that is costly or inconvenient for us. |
Future contract manufacturers are or will be subject to all of the risks and uncertainties that we would have if we manufactured the product candidates on our own. Similar to us, they are subject to ongoing, periodic, and unannounced inspection by the FDA and corresponding state and foreign agencies or their designees to ensure strict compliance with GMP regulations and other governmental regulations and corresponding foreign standards. Although we do not control compliance by our contract manufacturers with these regulations and standards, we—as the manufacturer—assume the liabilities for our contract manufacturers’ non-compliance. Our future contract manufacturers might not be able to comply with these regulatory requirements. If our third-party manufacturers fail to comply with applicable regulations, the FDA or other regulatory authorities could impose penalties on us, including fines, injunctions, civil penalties, consent decrees, compliance with FDA’s Application Integrity Policy, issuance of warning or untitled letters, denial of marketing approval of our future product candidates, delays, suspensions, or withdrawals of approvals, license revocation, seizures or recalls of product candidates or our other products, operating restrictions, and criminal prosecutions. Any of these actions could significantly and adversely affect supplies of our future product candidates or other products and could have a material adverse effect on our business, financial condition, and results of operations.
If we decide to use third-party manufacturers in the future, they will likely be dependent upon their own third-party suppliers, making us vulnerable to supply shortages and price fluctuations, which could harm our business.
The operations of any future third-party manufacturers will likely be dependent upon their own third-party suppliers. A supply interruption or an increase in demand beyond a supplier’s capabilities could harm the ability of any future manufacturers to manufacture our future product candidates or intended products until the manufacturer identifies and qualifies new sources of supply. Reliance on these third-party manufacturers and their suppliers could subject us to a number of risks that could harm our business, including:
| ● | interruption of supply resulting from modifications to or discontinuation of a supplier’s operations; |
| ● | failure of third-party manufacturers or suppliers to comply with their own legal and regulatory requirements; |
| ● | delays in product shipments resulting from uncorrected defects, reliability issues, or a supplier’s variation in a component; |
| ● | a lack of long-term supply arrangements for key components with our suppliers; |
| ● | inability to obtain adequate supply in a timely manner, or to obtain adequate supply on commercially reasonable terms; |
| ● | difficulty and cost associated with locating and qualifying alternative suppliers for components in a timely manner; |
| ● | production delays related to the evaluation and testing of products from alternative suppliers, and corresponding regulatory qualifications; |
| ● | delay in delivery due to suppliers prioritizing other customer orders over ours or those of our third-party manufacturers; |
| ● | damage to our brand reputation caused by defective components produced by the suppliers; and |
| ● | fluctuation in delivery by the suppliers due to changes in demand from us, our third-party manufacturers or their other customers. |
30
Any interruption in the supply of components of our future product candidates, or our inability to obtain substitute components or materials from alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demands of our clinical trials or of our future customers, which would have an adverse effect on our business.
We are subject to a multitude of manufacturing risks, any of which could substantially increase our costs and limit supply of our future product candidates.
The process of manufacturing our future product candidates is complex, highly regulated, and subject to several risks. For example, the process of manufacturing our future product candidates is extremely susceptible to product loss due to contamination, equipment failure or improper installation or operation of equipment, or vendor or operator error. Even minor deviations from normal manufacturing processes for any of our future product candidates could result in reduced production yields, product defects and other supply disruptions. If microbial, viral or other contaminations are discovered in our future product candidates or in the manufacturing facilities in which our future product candidates will be made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination. In addition, the manufacturing facilities in which our future product candidates will be made could be adversely affected by equipment failures, labor shortages, natural disasters, public health crises, pandemics and epidemics, such as the recent coronavirus disease 2019 (COVID-19), power failures and numerous other factors.
In addition, any adverse developments affecting manufacturing operations for our future product candidates may result in shipment delays, inventory shortages, lot failures, withdrawals or recalls or other interruptions in the supply of our future product candidates. We also may need to take inventory write-offs and incur other charges and expenses for future product candidates that fail to meet specifications, undertake costly remediation efforts or seek costlier manufacturing alternatives.
We will depend on third-party distributors in the future to market and sell our future product candidates which will subject us to a number of risks.
We will depend on third-party distributors to sell, market, and service our future product candidates in our intended markets. We are subject to a number of risks associated with reliance upon third-party distributors including:
| ● | lack of day-to-day control over the activities of third-party distributors; |
| ● | failure of the third-party distributors to comply with their own legal and regulatory requirements; |
| ● | third-party distributors may not commit the necessary resources to market and sell our future product candidates to our level of expectations; |
| ● | third-party distributors may terminate their arrangements with us on limited or no notice or may change the terms of these arrangements in a manner unfavorable to us; and |
| ● | disagreements with our future distributors could result in costly and time-consuming litigation or arbitration which we could be required to conduct in jurisdictions with which we are not familiar. |
If we fail to establish and maintain satisfactory relationships with our future third-party distributors, our revenues and market share may not grow as anticipated, and we could be subject to unexpected costs which could harm our results of operations and financial condition.
The successful commercialization of our future product candidates will depend on obtaining reimbursement from government and third-party payors.
If we successfully develop and obtain necessary regulatory approvals, we intend to sell our product candidates in countries such as the United States. In the United States, the market for any pharmaceutical product is affected by the availability of reimbursement from government and third-party payors, such as government health administration authorities, private health insurers, health maintenance organizations, and pharmacy benefit management companies. This, in turn, may make it more difficult for us to obtain adequate reimbursement from government and third-party payors, particularly if we cannot demonstrate a favorable cost-benefit relationship. Government and third-party payors may also deny coverage or offer inadequate levels of reimbursement for our potential products if they determine that the product has not received appropriate clearances from the FDA or other government regulators or is experimental, unnecessary or inappropriate.
31
In some other countries where we may seek to market our products, the pricing of prescription pharmaceutical products and services and the level of government reimbursement are subject to governmental control. In these countries, pricing negotiations with governmental authorities can take six to twelve months or longer after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we or our potential future collaborators may be required to conduct one or more clinical trials that compare the cost effectiveness of our product candidates or products to other available therapies. Conducting one or more additional clinical trials would be expensive and could result in delays in commercialization of our product candidates.
Managing and reducing health care costs has been a general concern of federal and state governments in the United States and various foreign governments. Although we do not believe that any recently enacted or presently proposed legislation in any jurisdictions in which we currently operate should impact our business based on our current model, we might be subject to future regulations or other cost-control initiatives that materially restrict the price we would receive for our products. In addition, government and third-party payors are increasingly challenging the price and cost-effectiveness of medical products and services, and many limit reimbursement for newly approved health care products. In particular, government and third-party payors may limit the indications for which they will reimburse patients who use any products that we may develop. Cost control initiatives could decrease the price for products that we may develop, which could result in lower product revenues to us.
We may enter into arrangements with third-party collaborators to help us develop our product candidates and commercialize our products, and our ability to commercialize such products may be impaired or delayed if collaborations are unsuccessful.
We are parties to various collaborations with third parties, and may enter into additional collaborations in the future. We are dependent upon the success of our current and any future collaborators in performing their responsibilities in connection with the relevant collaboration. If we fail to maintain these collaborative relationships for any reason, we would need to perform the activities that we currently anticipate would be performed by our collaborators on our own at our sole expense. This could substantially increase our capital needs, and we may not have the capability or financial capacity to undertake these activities on our own, or we may not be able to find other collaborators on acceptable terms, or at all. This may limit the programs we are able to pursue and result in significant delays in the development, sale, and manufacture of our future product candidates and products, and may have a material adverse effect on our business, financial condition, and results of operations.
Our dependence upon our current and potential future collaborations exposes us to a number of risks, including that our collaborators (i) may fail to cooperate or perform their contractual obligations, including financial obligations, (ii) may choose to undertake differing business strategies or pursue alternative technologies, or (iii) may take an opposing view regarding ownership of clinical trial results or intellectual property.
Due to these factors and other possible events, we could suffer delays in the research, development, or commercialization of our future product candidates or we may become involved in litigation or arbitration, which could be time consuming and expensive. We additionally may be compelled to split revenue with our collaborators, which could have a material adverse effect on our business, financial condition, and results of operations.
If we engage in future acquisitions or strategic partnerships, this may increase our capital requirements, dilute our stockholders, cause us to incur debt or assume contingent liabilities, and subject us to other risks.
From time to time, we may evaluate various acquisition opportunities and strategic partnerships, including licensing or acquiring complementary products or product candidates, intellectual property rights, technologies or businesses. Any potential acquisition or strategic partnership may entail numerous risks, including:
| ● | increased operating expenses and cash requirements; | |
| ● | the assumption of additional indebtedness or contingent liabilities; | |
| ● | the issuance of our equity securities; | |
| ● | assimilation of operations, intellectual property and products or product candidates of an acquired company, including difficulties associated with integrating new personnel; |
32
| ● | the diversion of our management’s attention from our existing programs and initiatives in pursuing such a strategic merger or acquisition; | |
| ● | retention of key employees, the loss of key personnel and uncertainties in our ability to maintain key business relationships; | |
| ● | risks and uncertainties associated with the other party to such a transaction, including the prospects of that party to receive marketing approvals for their existing products or product candidates; and | |
| ● | our inability to generate revenue from acquired technology, product candidates and/or products sufficient to meet our objectives in undertaking the acquisition or even to offset the associated acquisition and maintenance costs. |
A shortage of qualified registered nursing staff and other caregivers could adversely affect our partners’ ability to attract, train and retain qualified personnel and could increase operating costs.
Our clinics rely significantly on our partners’ ability to attract and retain caregivers who possess the skills, experience and licenses necessary to meet the requirements of our patients. We compete for personnel with other providers for qualified staff and caregivers. Our partners’ ability to attract and retain caregivers depends on several factors, including our partners’ ability to provide these caregivers with attractive assignments and competitive benefits and salaries. We cannot assure you that we will succeed in any of these areas. In addition, there are occasional shortages of qualified health care personnel in some of the markets in which we operate. As a result, we may face higher costs to attract caregivers and we may have to provide them with more attractive benefit packages than we originally anticipated, either of which could cause our profitability to decline. Finally, if we expand our operations into geographic areas where health care providers historically have unionized, we cannot assure you that negotiating collective bargaining agreements will not have a negative effect on our partners’ ability to timely and successfully recruit qualified personnel. Generally, if we are unable to attract and retain caregivers, the quality of our services may decline and we could lose patients and referral sources.
We anticipate generating revenue and profit margin under contracts with medical professional entities, and will face risks related to entering and retaining such contracts.
In our arrangements with separate legal professional entities (e.g., professional medical corporations) for providing business support services related to the infusion of ketamine, it is expected that our affiliated physicians will collect the fees for physician services provided. We cannot assure you that we will be successful in entering such contracts in a timely manner or at all due to issues related to the formation of such entities, which is currently underway in California and New York, or in retaining such contracts or that we will retain them on terms that are as favorable as present terms.
Any non-compete agreements and other restrictive covenants involving physicians may not be enforceable.
We anticipate entering into contracts with physicians and professional corporations in New York and California, and later in other states. Some of these contracts will include provisions preventing these physicians and professional corporations from engaging other business support services organizations both during and after the term of our relationship with them. The law governing non-compete agreements and other forms of restrictive covenants varies from state to state. Some states are reluctant to strictly enforce non-compete agreements and restrictive covenants applicable to physicians. There can be no assurance that our non-compete agreements will not be successfully challenged as unenforceable in certain states. In such event, we would be unable to prevent former affiliated physicians and professional corporations from engaging other business support services organizations that compete with us.
Failure of our affiliated physicians and other medical practitioners to comply with laws and regulations could result in suspension or revocation of our affiliated physicians’ licenses and termination of our service agreements with such affiliated physicians.
Our affiliated physicians are subject to various licensing laws and regulations relating to, among other things, the practice of medicine, adequacy of medical care, equipment, personnel and operating policies and procedures. Our affiliated physician practices may be subject to inspection by governmental and other authorities to assure continued compliance with the various standards necessary for licensing. Failure of our affiliated physicians and other medical practitioners to comply with these laws and regulations could result in suspension or revocation of our affiliated physicians’ licenses and termination of our service agreements with such affiliated physicians. While we have made reasonable efforts to ensure our affiliated physician practices substantially comply with licensing laws and regulations and standards, we cannot assure you that agencies that administer these programs will not find that the affiliated practices have failed to comply in some material respects. See “Business – Clinics” for further discussion regarding certain regulatory matters regarding the clinical infusion of ketamine to treat depression.
33
Risks Related to the Discovery, Development and Commercialization of Our Future Product Candidates
Interim, “topline” and preliminary data from our future clinical trials that we announce or publish from time to time may change as more data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we may publicly disclose preliminary or topline data from our preclinical studies and clinical trials, which is based on a preliminary analysis of then-available data. These results and related findings and conclusions are based on assumptions, estimations, calculations and conclusions, and are subject to change following the generation of additional data or a more comprehensive review of the data related to the particular study or trial. As a result, the topline or preliminary results that we report may differ from future results of the same studies, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated. Topline data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, topline and preliminary data should be viewed with caution until the final data are available.
From time to time, we may also disclose interim data from our preclinical studies and clinical trials. Interim data from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as subject enrollment continues and more subject data become available or as subjects from our clinical trials continue other treatments for their disease. Adverse differences between preliminary or interim data and final data could significantly harm our business prospects. Further, disclosure of interim data by us or by our competitors could result in volatility in the price of our Common Stock after this offering.
Further, others, including regulatory agencies, may not accept or agree with our assumptions, estimates, calculations, conclusions or analyses or may interpret or weigh the importance of data differently, which could impact the value of the particular program, the approvability or commercialization of the particular product candidate or product and our company in general. In addition, the information we choose to publicly disclose regarding a particular study or clinical trial is based on what is typically extensive information, and you or others may not agree with what we determine is material or otherwise appropriate information to include in our disclosure.
If the interim, topline, or preliminary data that we report differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for, and commercialize, our future product candidates may be harmed, which could have a material adverse effect on our business, financial condition, and results of operations.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on other product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we focus on research programs and product candidates that we identify for specific indications. As a result, we may forego or delay pursuit of opportunities with other therapeutic platforms or product candidates or for other indications that later prove to have greater commercial potential or a greater likelihood of success. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs, therapeutic platforms and product candidates for specific indications may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights.
34
The FDA and other comparable foreign regulatory authorities may not accept data from trials conducted in locations outside of their jurisdiction.
We may choose to conduct international clinical trials in the future. The acceptance of study data by the FDA or other comparable foreign regulatory authority from clinical trials conducted outside of their respective jurisdictions may be subject to certain conditions. In cases where data from foreign clinical trials are intended to serve as the basis for marketing approval in the United States, the FDA will generally not approve the application on the basis of foreign data alone unless (1) the data are applicable to the United States population and United States medical practice; (2) the trials are performed by clinical investigators of recognized competence; and (3) the FDA is able to validate the data through an on-site inspection or other appropriate means. The FDA may accept the use of some foreign data to support a marketing approval if the clinical trial meets certain requirements. Additionally, the FDA’s clinical trial requirements, including the adequacy of the subject population studied and statistical powering, must be met. Furthermore, such foreign trials would be subject to the applicable local laws of the foreign jurisdictions where the trials are conducted. There can be no assurance that the FDA or any applicable foreign regulatory authority will accept data from trials conducted outside of its respective jurisdiction. If the FDA or any applicable foreign regulatory authority does not accept such data, it would result in the need for additional trials, which would be costly and time-consuming and delay aspects of our business plan, and which may result in our future product candidates not receiving approval for commercialization in the applicable jurisdiction.
Obtaining and maintaining regulatory approval of a product in one jurisdiction does not mean that we will be successful in obtaining or maintaining regulatory approval in other jurisdictions.
Obtaining and maintaining regulatory approval of a product in one jurisdiction does not guarantee that we will be able to obtain or maintain regulatory approval in any other jurisdiction. For example, even if the FDA grants marketing approval of a product, comparable regulatory authorities in foreign jurisdictions must also approve the manufacturing, marketing and promotion and reimbursement of the product in those countries. However, a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in others. Moreover, product types or regulatory classifications, as well as approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from those in the United States, including different or additional preclinical studies or clinical trials, as clinical trials conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the United States, a product must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge for our products is also subject to approval.
Obtaining foreign regulatory approvals and establishing and maintaining compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our products in certain countries. If we or any future collaborator fails to comply with the regulatory requirements in international markets or fails to receive applicable marketing approvals, our target market will be reduced and our ability to realize the full market potential of our future product candidates will be harmed.
The FDA and other regulatory agencies actively enforce the laws and regulations prohibiting pre-approval promotion and the promotion of off-label uses.
The FDA prohibits the pre-approval promotion of drugs as safe and effective for the purposes for which they are under investigation. Similarly, the FDA prohibits the promotion of approved drugs for new or unapproved indications. If the FDA finds that we have engaged in pre-approval promotion of our future product candidates, or if any of our future product candidates are approved and we are found to have improperly promoted off-label uses of those products, we may become subject to significant liability. The FDA and other regulatory agencies strictly regulate the promotional claims that may be made about prescription products, such as our future product candidates, if approved. In particular, an approved product may not be promoted for uses that are not approved by the FDA or such other regulatory agencies as reflected in the product’s approved labeling. If we receive marketing approval for a product candidate, physicians may nevertheless prescribe it to their patients in a manner that is inconsistent with the approved label, which is within their purview as part of their practice of medicine. If we are found to have promoted such off-label uses, however, we may become subject to significant liability. The U.S. federal government has levied large civil and criminal fines against companies for alleged improper promotion of off-label use and has enjoined several companies from engaging in off-label promotion. The FDA has also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed. The FDA may also issue a public warning letter or untitled letter to the company. If we cannot successfully manage the promotion of our future approved products, we could become subject to significant liability, which would materially adversely affect our business and financial condition.
35
We may attempt to secure approval from the FDA or comparable foreign regulatory authorities through an expedited review program, and if we are unable to do so, then we could face increased expense to obtain, and delays in the receipt of, necessary marketing approvals.
We may in the future seek approval for one or more of our future product candidates under one of the FDA’s expedited review programs for serious conditions. These programs are available to sponsors of therapies that address an unmet medical need to treat a serious condition. The qualifying criteria and requirements vary for each expedited program. Prior to seeking review under one of these expedited programs for any of our future product candidates, we intend to seek feedback from the FDA and will otherwise evaluate our ability to seek and receive marketing approval through an expedited review program.
There can be no assurance that, after our evaluation of the FDA’s feedback and other factors, we will decide to pursue one or more of these expedited review programs. Similarly, there can be no assurance that after subsequent FDA feedback we will continue to pursue one or more of these expedited programs, even if we initially decide to do so. Furthermore, FDA could decide not to grant our request to use one or more of the expedited review programs for a product candidate, even if the FDA’s initial feedback is that the product candidate would qualify for such program(s). Moreover, FDA can decide to stop reviewing a product candidate under one or more of these expedited review programs if, for example, the conditions that warranted expedited review no longer apply to that product candidate.
Some of these expedited programs (e.g., accelerated approval) also require post-marketing clinical trials to be completed and, if any such required trial fails, the FDA could withdraw the approval of the product. If one of our future product candidates does not qualify for any expedited review program, then this could result in a longer time period to approval and commercialization of such product candidate, could increase the cost of development of such product candidate, and could harm our competitive position in the marketplace.
We may face difficulties from changes to current regulations and future legislation, both in the U.S. as well as in other foreign jurisdictions where we may be operating.
Existing regulations and regulatory policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our future product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability.
There have been judicial and congressional challenges to the Affordable Care Act. If a law is enacted, many if not all of the provisions of the PPACA may no longer apply to prescription drugs. While we are unable to predict what changes may ultimately be enacted, to the extent that future changes affect how any future products are paid for and reimbursed by government and private payers our business could be adversely impacted. On December 14, 2018, a federal district court in Texas ruled that the PPACA is unconstitutional as a result of the Tax Cuts and Jobs Act, the federal income tax reform legislation previously passed by Congress and signed by President Trump on December 22, 2017, that eliminated the individual mandate portion of the PPACA. The case, Texas, et al, v. United States of America, et al., (N.D. Texas), is an outlier, and the ruling has been stayed by the ruling judge, but in 2019, the Fifth Circuit Court of Appeals subsequently upheld the lower court decision which was then appealed to the United States Supreme Court. The U.S. Supreme Court declined to hear the appeal on an expedited basis and so no decision is expected until the next Supreme Court term in early 2021. We are not able to state with any certainty what will be the impact of this court decision on our business pending further court action and possible appeals. In November 2020, Joseph Biden was elected President and, in January 2021, the Democratic Party obtained control of the Senate. As a result of these electoral developments, it is unlikely that continued legislative efforts will be pursued to repeal PPACA. Instead, it is possible that legislation will be pursued to enhance or reform PPACA. We are not able to state with certainty what the impact of potential legislation will be on our business.
In addition, other legislative changes have been proposed and adopted in the United States that could impact our future business and operations, including those that may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on customers for our future product candidates, if approved, and accordingly, our business, financial condition, and results of operations.
36
Moreover, there has been heightened governmental scrutiny recently over the manner in which drug manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. For example, at the federal level, the Trump administration released a “Blueprint” to lower drug prices and reduce out of pocket costs of drugs that contains additional proposals to increase manufacturer competition, increase the negotiating power of certain federal healthcare programs, incentivize manufacturers to lower the list price of their products and reduce the out of pocket costs of drug products paid by consumers. Although future measures will require additional authorization to become effective, Congress and the Trump administration have each indicated that it will continue to seek new legislative and/or administrative measures to control drug costs. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
We expect that the ACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved product. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our future product candidates.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for biotechnology products. We cannot be sure whether additional legislative changes will be enacted, or whether FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our future product candidates, if any, may be. In addition, increased scrutiny by Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
Our relationships with healthcare professionals, clinical investigators, CROs and third-party payors in connection with our current and future business activities may be subject to federal and state healthcare fraud and abuse laws, false claims laws, transparency laws, government price reporting, and health information privacy and security laws, which could expose us to, among other things, criminal sanctions, civil penalties, contractual damages, exclusion from governmental healthcare programs, reputational harm, administrative burdens and diminished profits and future earnings.
Healthcare providers and third-party payors play a primary role in the recommendation and prescription of any future product candidates for which we obtain future marketing approval. Our current and future arrangements with healthcare professionals, clinical investigators, CROs, third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations include the following:
| ● | the federal Anti-Kickback Statute prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it in order to have committed a violation. In addition, the government may assert that a claim including items or services resulting from a violation of the U.S. federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act; |
| ● | the federal false claims and civil monetary penalties laws, including the civil False Claims Act, which can be enforced by private citizens through civil whistleblower or qui tam actions, prohibit individuals or entities from, among other things, knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; HIPAA, prohibits, among other things, executing or attempting to execute a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
37
| ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and their implementing regulations, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| ● | the federal Physician Payments Sunshine Act requires applicable manufacturers of covered drugs, devices and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to annually report to CMS starting in 2022 information regarding payments and other transfers of value to physicians, certain other healthcare providers and teaching hospitals, as well as information regarding ownership and investment interests held by physicians and their immediate family members. The information reported is publicly available on a searchable website, with disclosure required annually; and |
| ● | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers. |
Some state laws require biotechnology companies to comply with the biotechnology industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and may require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures. Some state laws require biotechnology companies to report information on the pricing of certain drug products. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. For instance, the collection and use of health data in the United Kingdom and the European Union is governed by the GDPR (and in the United Kingdom, retained GDPR following Brexit), which extends the geographical scope of European Union data protection law to non-European Union entities under certain conditions, tightens existing European Union data protection principles, creates new obligations for companies and new rights for individuals. Failure to comply with the GDPR may result in substantial fines and other administrative penalties. In addition, on June 28, 2018, the State of California enacted the California Consumer Privacy Act, or CCPA, which took effect on January 1, 2020. The CCPA creates individual privacy rights for California consumers and increases the privacy and security obligations of entities handling certain personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. The CCPA may increase our compliance costs and potential liability, and similar laws have been proposed at the federal level and in other states.
Efforts to ensure that our current and future business arrangements with third parties will comply with applicable healthcare laws and regulations will involve on-going substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant penalties, including civil, criminal and administrative penalties, damages, fines, disgorgement, individual imprisonment, exclusion from participation in government funded healthcare programs, such as Medicare and Medicaid, integrity oversight and reporting obligations, temporary or permanent debarment, contractual damages, reputational harm, diminished profits and future earnings and the curtailment or restructuring of our operations. Defending against any such actions can be costly, time-consuming and may require significant financial and personnel resources. Therefore, even if we are successful in defending against any such actions that may be brought against us, our business may be impaired. Further, if any of the physicians or other healthcare providers or entities with whom we expect to do business are found not to be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Inadequate funding for the FDA and other government agencies, or future government shutdown and or furlough of government employees, or public health emergencies could hinder their ability to hire and retain key leadership and other personnel, prevent new products and services from being reviewed or approved in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel, the availability of industry-paid user fees, and statutory, regulatory, and policy changes. Average review times for product approvals at the FDA have fluctuated in recent years as a result. In addition, government funding of other government agencies on which our operations may rely, including those that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
38
Disruptions at the FDA and other agencies, including those resulting from the current COVID-19 global pandemic, may also slow the time necessary for new products to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, if a prolonged government shutdown and/or government employee furloughs were to occur, or if FDA’s response to a global pandemic such as COVID-19 diverts FDA resources and attention to other regulatory efforts, then the ability of the FDA to timely review and process our regulatory submissions could be significantly impacted, which could have a material adverse effect on our business, financial condition, and results of operations. Further, upon completion of this offering and in our operations as a public company, future government shutdowns, furloughs or public health emergencies could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on our business, financial condition, and results of operations.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of hazardous and flammable materials, including chemicals.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or commercialization efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Our research and development activities could be affected or delayed as a result of possible restrictions on animal testing.
Certain laws and regulations will require us to test our future product candidates on animals before initiating clinical trials involving humans. Animal testing activities have been the subject of controversy and adverse publicity. Animal rights groups and other organizations and individuals have attempted to stop animal testing activities by pressing for legislation and regulation in these areas and by disrupting these activities through protests and other means. To the extent the activities of these groups are successful, or if the laws and regulations regarding animal testing otherwise change, our research and development activities may be interrupted, delayed or become more expensive.
Our business activities may be subject to the U.S. Foreign Corrupt Practices Act, or the FCPA, and similar anti-bribery and anti-corruption laws of other countries in which we operate, as well as U.S. and certain foreign export controls, trade sanctions, and import laws and regulations. Compliance with these legal requirements could limit our ability to compete in foreign markets and subject us to liability if we violate them.
If we further expand our operations outside of the United States, we must dedicate additional resources to comply with numerous laws and regulations in each jurisdiction in which we plan to operate. Our business activities may be subject to the FCPA and similar anti-bribery or anti-corruption laws, regulations or rules of other countries in which we operate. The FCPA generally prohibits companies and their employees and third party intermediaries from offering, promising, giving or authorizing the provision of anything of value, either directly or indirectly, to a non-U.S. government official in order to influence official action or otherwise obtain or retain business. The FCPA also requires public companies to make and keep books and records that accurately and fairly reflect the transactions of the corporation and to devise and maintain an adequate system of internal accounting controls. Our business is heavily regulated and therefore involves significant interaction with public officials, including officials of non-U.S. governments. Additionally, in many other countries, hospitals owned and operated by the government, and doctors and other hospital employees would be considered foreign officials under the FCPA. Recently the Securities and Exchange Commission (SEC) and Department of Justice (DOJ) have increased their FCPA enforcement activities with respect to biotechnology and pharmaceutical companies. There is no certainty that all of our employees, agents or contractors, or those of our affiliates, will comply with all applicable laws and regulations, particularly given the high level of complexity of these laws. Violations of these laws and regulations could result in fines, criminal sanctions against us, our officers or our employees, disgorgement, and other sanctions and remedial measures, and prohibitions on the conduct of our business. Any such violations could include prohibitions on our ability to offer our products in one or more countries and could materially damage our reputation, our brand, our international activities, our ability to attract and retain employees and our business, prospects, operating results and financial condition.
39
In addition, our products and technology may be subject to U.S. and foreign export controls, trade sanctions and import laws and regulations. Governmental regulation of the import or export of our products and technology, or our failure to obtain any required import or export authorization for our products, when applicable, could harm our international sales and adversely affect our revenue. Compliance with applicable regulatory requirements regarding the export of our products may create delays in the introduction of our products in international markets or, in some cases, prevent the export of our products to some countries altogether. Furthermore, U.S. export control laws and economic sanctions prohibit the shipment of certain products and services to countries, governments, and persons targeted by U.S. sanctions. If we fail to comply with export and import regulations and such economic sanctions, penalties could be imposed, including fines and/or denial of certain export privileges. Moreover, any new export or import restrictions, new legislation or shifting approaches in the enforcement or scope of existing regulations, or in the countries, persons, or products targeted by such regulations, could result in decreased use of our products by, or in our decreased ability to export our products to existing or potential customers with international operations. Any decreased use of our products or limitation on our ability to export or sell access to our products would likely adversely affect our business.
Risks Related to Employee Matters, Managing Our Growth and Other Risks Related to Our Business
We have never commercialized a product candidate before and may lack the necessary expertise, personnel and resources to successfully commercialize any products on our own or together with suitable collaborators.
We have never commercialized a product candidate, and we currently have no sales force, marketing or distribution capabilities, nor do any of our current employees have any experience in commercializing a regulated product. To achieve commercial success for our future product candidates, which we may license to others, we will rely on the assistance and guidance of those collaborators. For product candidates for which we retain commercialization rights, we will have to develop our own sales, marketing and supply organization or outsource these activities to a third party.
Factors that may affect our ability to commercialize our future approved products on our own include recruiting and retaining adequate numbers of effective sales and marketing personnel, obtaining access to or persuading adequate numbers of physicians to prescribe our products and other unforeseen costs associated with creating an independent sales and marketing organization. Developing a sales and marketing organization will be expensive and time-consuming and could delay the launch of our future approved products. We may not be able to build an effective sales and marketing organization. If we are unable to build our own distribution and marketing capabilities or to find suitable partners for the commercialization of our future approved products, we may not generate revenues from them or be able to reach or sustain profitability.
In order to successfully implement our plans and strategies, we will need to grow our organization, and we may experience difficulties in managing this growth.
As of December 31, 2020, we had two part time employees and one full time employee, in addition to Zen Health’s staff of over 60 team members across three clinics. In order to successfully implement our development and commercialization plans and strategies, and as we transition into operating as a public company, we expect to need additional managerial, operational, sales, marketing, financial and other personnel. Future growth would impose significant added responsibilities on members of management, including:
| ● | identifying, recruiting, integrating, maintaining and motivating additional employees; | |
| ● | managing our internal development efforts effectively, including preclinical and clinical studies and investigations, as well as FDA and other comparable foreign regulatory agencies’ review process for any current or future product candidates, while complying with any contractual obligations to contractors and other third parties we may have; and | |
| ● | improving our operational, financial and management controls, reporting systems and procedures. |
Our future financial performance and our ability to successfully develop and, if approved, commercialize, any current or future product candidates will depend, in part, on our ability to effectively manage any future growth, and our management may also have to divert a disproportionate amount of its attention away from day-to-day activities in order to devote a substantial amount of time to managing these growth activities.
40
We currently rely, and for the foreseeable future will continue to rely, in substantial part on certain independent organizations, advisors and consultants to provide certain services, including key aspects of clinical development and manufacturing. We cannot assure you that the services of independent organizations, advisors and consultants will continue to be available to us on a timely basis when needed, or that we can find qualified replacements. In addition, if we are unable to effectively manage our outsourced activities or if the quality or accuracy of the services provided by third party service providers is compromised for any reason, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain marketing approval of our current and future product candidates or otherwise advance our business. We cannot assure you that we will be able to manage our existing third-party service providers or find other competent outside contractors and consultants on economically reasonable terms, or at all.
If we are not able to effectively expand our organization by hiring new employees and/or engaging additional third party service providers, we may not be able to successfully implement the tasks necessary to further develop and commercialize our current and future product candidates and, accordingly, may not achieve our research, development and commercialization goals.
We cannot assure you that our plans to raise capital will be successful.
As of December 31, 2020, we had working capital of approximately $241,355. Management’s plans to address this need for capital through this offering are discussed in the section of this prospectus titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” We cannot assure you that our plans to raise capital will be successful. These factors, among others, raise substantial doubt about our ability to continue as a going concern. The financial statements contained elsewhere in this prospectus do not include any adjustments that might result from our inability to consummate this offering or our inability to continue as a going concern.
Risks Related to this Offering and Ownership of Our Common Stock
There has been no prior public market for our Common Stock. We do not know whether an active, liquid and orderly trading market will develop for our Common Stock or what the market price of our Common Stock will be and as a result it may be difficult for you to sell your shares of our Common Stock.
Prior to this offering, no public market for shares of our Common Stock existed and an active trading market for our Common Stock may never develop or be sustained following this offering. We will determine the initial public offering price for our Common Stock through negotiations with the underwriters, and the negotiated price may not be indicative of the market price of our Common Stock after this offering. The market value of our Common Stock may decrease from the initial public offering price. As a result of these and other factors, you may be unable to resell your shares of our Common Stock at or above the initial public offering price. The lack of an active market may impair your ability to sell your shares at the time you wish to sell them or at a price that you consider reasonable. The lack of an active market may also reduce the fair market value of your shares. Furthermore, an inactive market may also impair our ability to raise capital by selling shares of our Common Stock and may impair our ability to enter into strategic collaborations or acquire companies, technologies or other assets by using our shares of Common Stock as consideration.
The price of our stock may be volatile, and you could lose all or part of your investment.
The trading price of our Common Stock following this offering is likely to be highly volatile and subject to wide fluctuations in response to various factors, some of which we cannot control. The stock market in general, and pharmaceutical and biotechnology companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies.
Broad market and industry factors may negatively affect the market price of our Common Stock, regardless of our actual operating performance. In addition to the factors discussed in this “Risk Factors” section and elsewhere in this prospectus, these factors include:
| ● | the timing and results of preclinical studies and clinical trials of our future product candidates or those of our competitors; | |
| ● | the success of competitive products or announcements by potential competitors of their product development efforts; |
41
| ● | regulatory actions with respect to our or our competitors’ product candidates or products; | |
| ● | actual or anticipated changes in our growth rate relative to our competitors; | |
| ● | regulatory or legal developments in the United States and other countries; | |
| ● | developments or disputes concerning patent applications, issued patents or other proprietary rights; | |
| ● | the recruitment or departure of key personnel; |
| ● | announcements by us or our competitors of significant acquisitions, strategic collaborations, joint ventures, or capital commitments; | |
| ● | actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; | |
| ● | fluctuations in the valuation of companies perceived by investors to be comparable to us; | |
| ● | market conditions in the pharmaceutical and biotechnology sector; | |
| ● | changes in the structure of healthcare payment systems; | |
| ● | share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; | |
| ● | announcement or expectation of additional financing efforts; | |
| ● | sales of our Common Stock by us, our insiders or our other stockholders; | |
| ● | expiration of market stand-off or lock-up agreements; and | |
| ● | general economic, industry and market conditions. |
The realization of any of the above risks or any of a broad range of other risks, including those described in this “Risk Factors” section, could have a dramatic and adverse impact on the market price of our Common Stock.
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our future product candidates on unfavorable terms to us.
In order to meet our operational goals, we will need to obtain additional capital, which we will likely obtain through a variety of means, including through public or private equity, debt financings or other sources, including up-front payments and milestone payments from strategic collaborations. To the extent that we raise additional capital through the sale of convertible debt or equity securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a stockholder. Such financing may result in dilution to stockholders, imposition of debt covenants, increased fixed payment obligations or other restrictions that may affect our business. If we raise additional funds through up-front payments or milestone payments pursuant to strategic collaborations with third parties, we may have to relinquish valuable rights to our future product candidates, or grant licenses on terms that are not favorable to us. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
42
If securities or industry analysts do not publish research or reports, or if they publish adverse or misleading research or reports, regarding us, our business or our market, our stock price and trading volume could decline.
The trading market for our Common Stock will be influenced by the research and reports that securities or industry analysts publish about us, our business or our market. We do not currently have and may never obtain research coverage by securities or industry analysts. If no or few securities or industry analysts commence coverage of us, the stock price would be negatively impacted. In the event we obtain securities or industry analyst coverage, if any of the analysts who cover us issue adverse or misleading research or reports regarding us, our business model, our intellectual property, our stock performance or our market, or if our operating results fail to meet the expectations of analysts, our stock price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
Our quarterly operating results may fluctuate significantly or may fall below the expectations of investors or securities analysts, each of which may cause our stock price to fluctuate or decline.
We expect our operating results to be subject to quarterly fluctuations. Our net loss and other operating results will be affected by numerous factors, including:
| ● | variations in the level of expense related to the ongoing development of our future product candidates or future development programs; | |
| ● | results of clinical trials, or the addition or termination of clinical trials or funding support by us or potential future partners; | |
| ● | our execution of any collaboration, licensing or similar arrangements, and the timing of payments we may make or receive under potential future arrangements or the termination or modification of any such potential future arrangements; | |
| ● | any intellectual property infringement, misappropriation or violation lawsuit or opposition, interference or cancellation proceeding in which we may become involved; | |
| ● | additions and departures of key personnel; | |
| ● | strategic decisions by us or our competitors, such as acquisitions, divestitures, spin-offs, joint ventures, strategic investments or changes in business strategy; | |
| ● | if any of our future product candidates receive regulatory approval, the terms of such approval and market acceptance and demand for such approved products; | |
| ● | regulatory developments affecting our future product candidates, or those of our competitors; and | |
| ● | changes in general market and economic conditions. |
If our quarterly operating results fall below the expectations of investors or securities analysts, the price of our Common Stock could decline substantially. Furthermore, any quarterly fluctuations in our operating results may, in turn, cause the price of our stock to fluctuate substantially. We believe that quarterly comparisons of our financial results are not necessarily meaningful and should not be relied upon as an indication of our future performance.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, stockholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our Common Stock.
Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any testing by us conducted in connection with Section 404 of the Sarbanes-Oxley Act, or any subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our financial statements or identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our stock.
43
We will be required to disclose changes made in our internal controls and procedures on a quarterly basis and our management will be required to assess the effectiveness of these controls annually. However, for as long as we are an emerging growth company, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal controls over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act. We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of our initial public offering; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the SEC. An independent assessment of the effectiveness of our internal controls over financial reporting could detect problems that our management’s assessment might not. Undetected material weaknesses in our internal controls over financial reporting could lead to restatements of our financial statements and require us to incur the expense of remediation.
If you purchase shares of our Common Stock in our initial public offering, you will experience substantial and immediate dilution.
The initial public offering price of $ per share is substantially higher than the net tangible book value per share of our outstanding Common Stock immediately following the completion of this offering. If you purchase shares of Common Stock in this offering, you will experience substantial and immediate dilution in the pro forma net tangible book value per share of $ per share as of December 31, 2020. That is because the price that you pay will be substantially greater than the pro forma net tangible book value per share of the Common Stock that you acquire. This dilution is due in large part to the fact that our earlier investors paid substantially less than the initial public offering price when they purchased their shares of our capital stock. You will experience additional dilution when those holding derivative securities or warrants vest or exercise their right to purchase Common Stock under our equity incentive plans or when we otherwise issue additional shares of Common Stock. See “Dilution.”
Sales of a substantial number of shares of our Common Stock in the public market could cause our stock price to fall.
Our Common Stock price could decline as a result of sales of a large number of shares of Common Stock after this offering or the perception that these sales could occur. These sales, or the possibility that these sales may occur, might also make it more difficult for us to sell equity securities in the future at a time and price that we deem appropriate.
Upon the completion of this offering, shares of Common Stock will be outstanding ( shares if the underwriters exercise their option to purchase additional shares from us in full), based on the number of shares outstanding as of , 2021.
All shares of Common Stock expected to be sold in this offering will be freely tradable without restriction or further registration under the Securities Act unless held by our “affiliates” as defined in Rule 144 under the Securities Act. Shares issued upon the exercise of stock options and warrants outstanding under our equity incentive plans or pursuant to future awards granted under those plans will become available for sale in the public market to the extent permitted by the provisions of applicable vesting schedules, market stand-off agreements and/or lock-up agreements, as well as Rules 144 and 701 under the Securities Act. For more information, see “Shares Eligible for Future Sale.”
We intend to register the offer and sale of all shares of Common Stock that we may issue under our equity compensation plans. Once we register the offer and sale of shares for the holders of registration rights and shares that may be issued under our equity incentive plans, these shares will be able to be sold in the public market upon issuance, subject to the lock-up agreements described under “Underwriting.”
In addition, in the future, we may issue additional shares of Common Stock, or other equity or debt securities convertible into Common Stock, in connection with a financing, acquisition, employee arrangement or otherwise. Any such issuance could result in substantial dilution to our existing stockholders and could cause the price of our Common Stock to decline.
We are an “emerging growth company,” and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our Common Stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we intend to take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including:
| ● | being permitted to provide only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure in this prospectus; | |
| ● | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act; |
44
| ● | not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements; | |
| ● | reduced disclosure obligations regarding executive compensation in this prospectus and our periodic reports and proxy statements; and | |
| ● | exemptions from the requirements of holding nonbinding advisory stockholder votes on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
We cannot predict if investors will find our Common Stock less attractive because we may rely on these exemptions. If some investors find our Common Stock less attractive as a result, there may be a less active trading market for our Common Stock and our stock price may be more volatile.
We will remain an emerging growth company until the earliest to occur of: (1) the last day of the fiscal year in which we have more than $1.07 billion in annual revenue; (2) the date we qualify as a “large accelerated filer,” with at least $700 million of equity securities held by non-affiliates; (3) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period; and (4) the last day of the fiscal year ending after the fifth anniversary of our initial public offering.
Pursuant to the JOBS Act, as an emerging growth company, we have elected to use the extended transition period for complying with any new or revised financial accounting standards to delay adopting new or revised accounting standards until such time as those standards apply to private companies.
The requirements of being a public company may strain our resources, result in more litigation and divert management’s attention.
As a public company, we will be subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, or the Dodd-Frank Act, the listing requirements of Nasdaq and other applicable securities rules and regulations. Complying with these rules and regulations will increase our legal and financial compliance costs, make some activities more difficult, time consuming or costly and increase demand on our systems and resources, including management. The Exchange Act requires, among other things, that we file annual, quarterly and current reports with respect to our business and operating results. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. We are required to disclose changes made in our internal control and procedures on a quarterly basis. In order to maintain and, if required, improve our disclosure controls and procedures and internal control over financial reporting to meet this standard, significant resources and management oversight may be required. As a result, management’s attention may be diverted from other business concerns, which could adversely affect our business and operating results. We may also need to hire additional employees or engage outside consultants to comply with these requirements, which will increase our costs and expenses.
In addition, changing laws, regulations and standards relating to corporate governance and public disclosure are creating uncertainty for public companies, increasing legal and financial compliance costs and making some activities more time consuming. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management’s time and attention from revenue-generating activities to compliance activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to their application and practice, regulatory authorities may initiate legal proceedings against us and our business may be adversely affected.
These new rules and regulations may make it more expensive for us to obtain director and officer liability insurance and, in the future, we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified members of our board of directors, particularly to serve on our audit committee and compensation committee, and qualified executive officers.
45
By disclosing information in this prospectus and in future filings required of a public company, our business and financial condition will become more visible, which we believe may result in threatened or actual litigation, including by competitors and other third parties. If those claims are successful, our business could be seriously harmed. Even if the claims do not result in litigation or are resolved in our favor, the time and resources needed to resolve them could divert our management’s resources and seriously harm our business.
We may be subject to securities litigation, which is expensive and could divert management attention.
The market price of our Common Stock may be volatile and, in the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business.
We do not currently intend to pay dividends on our Common Stock and, consequently, your ability to achieve a return on your investment will depend on appreciation of the value of our Common Stock.
We have never declared or paid any cash dividends on our equity securities. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Any return to stockholders will therefore be limited to any appreciation in the value of our Common Stock, which is not certain.
Provisions in our certificate of incorporation and bylaws and Delaware law might discourage, delay or prevent a change in control of our company or changes in our management and, therefore, depress the market price of our Common Stock.
Our certificate of incorporation and bylaws contain provisions that could depress the market price of our Common Stock by acting to discourage, delay or prevent a change in control of our company or changes in our management that the stockholders of our Company may deem advantageous. These provisions, among other things:
| ● | prohibit cumulative voting; | |
| ● | authorize our board of directors to amend the bylaws; and | |
| ● | establish advance notice requirements for nominations for election to our board or for proposing matters that can be acted upon by stockholders at annual stockholder meetings. | |
In addition, Section 203 of the General Corporation Law of the State of Delaware, or the DGCL, prohibits a publicly-held Delaware corporation from engaging in a business combination with an interested stockholder, generally a person which together with its affiliates owns, or within the last three years has owned, 15% of our voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner.
Any provision of our certificate of incorporation, bylaws or Delaware law that has the effect of delaying or preventing a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our capital stock and could also affect the price that some investors are willing to pay for our Common Stock.
There is no guarantee that our Common Stock will be listed on Nasdaq.
We intend to apply to have our shares of Common Stock listed on The Nasdaq Capital Market. Upon completion of this offering, we believe that we will satisfy the listing requirements and expect that our Common Stock will be listed on The Nasdaq Capital Market. Such listing, however, is not guaranteed. If the application is not approved, we will seek to have our Common Stock quoted on the OTCQB maintained by the OTC Markets Group, Inc. Even if such listing is approved, there can be no assurance any broker will be interested in trading our Common Stock. Therefore, it may be difficult to sell any shares you purchase in this offering if you desire or need to sell them. Our lead underwriter is not obligated to make a market in our Common Stock, and even after making a market, can discontinue market making at any time without notice. Neither we nor the underwriters can provide any assurance that an active and liquid trading market in our Common Stock will develop or, if developed, that the market will continue.
46
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that can involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this prospectus, including statements regarding our future results of operations and financial position, business strategy, prospective products, product approvals, research and development costs, future revenue, timing and likelihood of success, plans and objectives of management for future operations, future results of anticipated products and prospects, plans and objectives of management are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
In some cases, you can identify forward-looking statements by terms such as “anticipate,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” ,” “potential,” “predict,” “project,” “should,” “target,” “will,” or “would” or the negative of these terms or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements contained in this prospectus include, but are not limited to, statements about:
| ● | the ability of our clinical trials to demonstrate safety and efficacy of our future product candidates, and other positive results; | |
| ● | the timing and focus of our future preclinical studies and clinical trials, and the reporting of data from those studies and trials; | |
| ● | the size of the market opportunity for our future product candidates, including our estimates of the number of patients who suffer from the diseases we are targeting; | |
| ● | the success of competing therapies that are or may become available; | |
| ● | the beneficial characteristics, safety, efficacy and therapeutic effects of our future product candidates; | |
| ● | our ability to obtain and maintain regulatory approval of our future product candidates; | |
| ● | our plans relating to the further development of our future product candidates, including additional disease states or indications we may pursue; | |
| ● | existing regulations and regulatory developments in the United States and other jurisdictions; | |
| ● | our plans and ability to obtain or protect intellectual property rights, including extensions of existing patent terms where available and our ability to avoid infringing the intellectual property rights of others; | |
| ● | the need to hire additional personnel and our ability to attract and retain such personnel; | |
| ● | our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; | |
| ● | our dependence on third parties; | |
| ● | our financial performance; |
47
| ● | the period over which we estimate our existing cash and cash equivalents will be sufficient to fund our future operating expenses and capital expenditure requirements; | |
| ● | our ability to generate revenue and profit margin under our anticipated contracts which is subject to certain risks; | |
| ● | difficulties in our and our partners’ ability to recruit and retain qualified physicians and other healthcare professionals, and enforce our non-compete agreements with our physicians; and | |
| ● | our ability to restructure our operations to comply with future changes in government regulation. |
We have based these forward-looking statements largely on our current expectations and projections about our business, the industry in which we operate and financial trends that we believe may affect our business, financial condition, results of operations and prospects, and these forward-looking statements are not guarantees of future performance or development. These forward-looking statements speak only as of the date of this prospectus and are subject to a number of risks, uncertainties and assumptions described in the section titled “Risk Factors” and elsewhere in this prospectus. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein whether as a result of any new information, future events or otherwise.
In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this prospectus, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and you are cautioned not to unduly rely upon these statements.
48
This prospectus contains industry, market and competitive position data from our own internal estimates and research as well as industry and general publications and research surveys and studies conducted by third parties. Industry publications, studies and surveys generally state that they have been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. Our internal data and estimates are based upon information obtained from trade and business organizations and other contacts in the markets in which we operate and our management’s understanding of industry conditions. While we believe that each of these studies and publications is reliable, we have not independently verified market and industry data from third-party sources. While we believe our internal company research is reliable and the market definitions are appropriate, neither such research nor definitions have been verified by an independent source.
The industry in which we operate is subject to risks and uncertainties due to a variety of factors, including those described in the section titled “Risk Factors.” These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.
49
We estimate that the net proceeds to us from this offering will be approximately $ million, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. If the underwriters’ option to purchase additional shares from us is exercised in full, we estimate that our net proceeds will be approximately $ million. Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share would increase (decrease) the net proceeds to us from this offering by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. Each increase (decrease) of 100,000 in the number of shares we are offering would increase (decrease) the net proceeds to us from this offering, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, by $ million, assuming the assumed initial public offering price stays the same.
The principal purposes of this offering are to increase our capitalization and financial flexibility, to create a public market for our Common Stock and to facilitate our future access to the capital markets. As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds we receive from this offering. However, we currently intend to use the net proceeds we receive from this offering as follows:
| ● | approximately $ million to fund research and development, including clinical trials and product development for our existing pipeline; |
| ● | approximately $ million to invest in developing our U.S. clinic and UK clinic business; and |
| ● | the remainder for intellectual property, business costs, working capital and selling, general and administrative purposes. |
We will have broad discretion over how to use the net proceeds we receive from this offering. We intend to invest the net proceeds we receive from this offering that are not used as described above in a variety of capital preservation investments, including short-term, investment-grade, interest-bearing instruments and U.S. government securities.
This expected use of the net proceeds from this offering represents our intentions based upon our current plans and business conditions, which could change in the future as our plans and business conditions evolve. We may also use a portion of the net proceeds to in-license, acquire or invest in additional businesses, technologies, products or assets, although currently we have no specific agreements, commitments or understandings in this regard. As of the date of this prospectus, we cannot predict with certainty all of the particular uses for the net proceeds to be received upon the closing of this offering or the amounts that we will actually spend on the uses set forth above. Predicting the cost necessary to develop product candidates can be difficult and we anticipate that we will need additional funds to complete the development of any product candidates we identify. The amounts and timing of our actual expenditures and the extent of clinical development may vary significantly depending on numerous factors, including the progress of our development efforts, the status of and results from pre-clinical studies and any clinical trials we may commence in the future, as well as any collaborations that we may enter into with third parties for our future product candidates and any unforeseen cash needs. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering.
We believe that the net proceeds from this offering, together with our existing cash and cash equivalents, will enable us to fund our operating expenses and capital expenditure requirements through second half of 2022. We have based this estimate on assumptions that may prove to be incorrect, and we could use our available capital resources sooner than we currently expect. We may satisfy our future cash needs through the sale of equity securities, debt financings, working capital lines of credit, corporate collaborations or license agreements, grant funding, interest income earned on invested cash balances or a combination of one or more of these sources.
50
We have never declared or paid any cash dividends on our capital stock. We intend to retain future earnings, if any, to finance the operation and expansion of our business and do not anticipate paying any cash dividends in the foreseeable future. Any future determination related to our dividend policy will be made at the discretion of our board of directors after considering our financial condition, results of operations, capital requirements, business prospects and other factors the board of directors deems relevant, and subject to the restrictions contained in any future financing instruments.
51
The following table sets forth our cash and cash equivalents and capitalization as of December 31, 2020, as follows:
| ● | on an actual basis; | |
| ● | on a pro forma basis to give effect to the issuance of an aggregate of 635,594 shares of our common stock in January 2021 for aggregate net proceeds of $1,209,000; and | |
| ● | on an pro forma as adjusted basis to give further effect to our issuance and sale of shares of our Common Stock in this offering at an assumed initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us and therefore providing net proceeds of approximately $ million. | |
Information below on a pro forma as adjusted basis is illustrative only, and our capitalization following the closing of this offering will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing. You should read this information in conjunction with our financial statements and the related notes included elsewhere in this prospectus and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section and other financial information contained in this prospectus.
| As of December 31, 2020 | ||||||||||||
| Actual | Pro Forma | Pro Forma As Adjusted(1) | ||||||||||
| Cash and cash equivalents | $ | 243,650 | $ | 13,277,366 | ||||||||
| Stockholders’ Equity | ||||||||||||
| Preferred stock, par value $0.0001 per share: 5,000,000 shares authorized 0 shares issued and outstanding | - | - | ||||||||||
| Common Stock, par value $0.0001 per share: 495,000,000 shares authorized 7,300,000 shares issued and outstanding | 14,938 | 16,438 | ||||||||||
| Additional paid-in capital | 267,401 | 13,301,912 | ||||||||||
| Accumulated deficit | (40,984 | ) | (40,984 | ) | ||||||||
| Total equity | $ | 241,355 | $ | 13,277,366 | ||||||||
| Total capitalization | $ | 241,355 | $ | 13,277,366 | ||||||||
| (1) | Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) the pro forma as adjusted amount of each of cash and cash equivalents, total equity and total capitalization by $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase (decrease) of 100,000 shares in the number of shares offered by us at the assumed initial public offering price per share, which is the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) the pro forma as adjusted amount of each of cash and cash equivalents, total equity and total capitalization by approximately $ million. |
The number of shares of our Common Stock on a pro forma as adjusted basis set forth in the table above is based on 7,300,000 shares of our Common Stock outstanding as of December 31, 2020, and excludes:
● |
shares of Common Stock issuable upon exercise of warrants to be issued to the representative of the underwriters as part of this offering at an exercise price of $ (assuming an initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus)). |
52
If you invest in our Common Stock in this offering, your ownership interest will be immediately diluted to the extent of the difference between the assumed initial public offering price of $ per share (the midpoint of the range appearing on the front cover of this prospectus) and the as adjusted net tangible book value per share of our Common Stock immediately upon the consummation of this offering. Net tangible book value per share represents the book value of our tangible assets less the book value of our total liabilities divided by the number of shares of Common Stock then issued and outstanding.
Our net tangible book value as of , 2021 was $ million, or $ per share, based on an assumed initial public offering price of $ per share (the midpoint of the range appearing on the front cover of this prospectus). After giving effect to our sale of shares of Common Stock in this offering at an assumed initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, our as adjusted net tangible book value as of , 2021 would have been approximately $ , or approximately $ per share (assuming no exercise of the underwriters’ option to purchase additional shares of our Common Stock). This amount represents an immediate and substantial dilution of $ per share to new investors purchasing Common Stock in this offering. The following table illustrates this dilution per share:
| Assumed initial public offering price per share | $ | |||
| Net tangible book value per share as of , 2021 | $ | |||
| Increase in net tangible book value per share attributable to this offering | $ | |||
| As adjusted net tangible book value per share after giving effect to this offering | $ | |||
| Dilution per share to new investors participating in this offering | $ |
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share (the midpoint of the range appearing on the front cover of this prospectus) would increase (decrease) the as adjusted net tangible book value by approximately $ million, or approximately $ per share, and increase (decrease) the dilution per share to new investors by approximately $ per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. An increase of 100,000 shares in the number of shares offered by us would increase our as adjusted net tangible book value by approximately $ million, or $ per share and the dilution per share to investors purchasing Common Stock in this offering would be $ per share, assuming the assumed initial public offering price remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, a decrease of 100,000 shares in the number of shares offered by us would decrease our as adjusted net tangible book value by approximately $ million, or $ per share and the dilution per share to investors purchasing Common Stock in this offering would be $ per share, assuming the assumed initial public offering price remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. The as adjusted information discussed above is illustrative only and will change based on the actual initial public offering price and other terms of this offering determined at pricing.
If the underwriters exercise their option in full to purchase additional shares of our Common Stock in this offering, the as adjusted net tangible book value per share after this offering would be $ per share, and the as adjusted dilution to new investors would be $ per share, in each case assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and after deducting underwriting discounts and commissions and the estimated offering expenses payable by us.
The following table summarizes, on an as adjusted basis described above, as of , 2021, the differences between the number of shares of Common Stock purchased from us, the total consideration paid and the average price per share paid by existing stockholders and by new investors participating in this offering at an assumed initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, before deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. As the table shows, new investors purchasing Common Stock in this offering will pay an average price per share substantially higher than our existing stockholders paid.
53
(In thousands, except per share amounts and percentages)
| Shares Purchased | Total Consideration | Average Share | ||||||||||||||||||
| Number | Percent | Amount | Percent | Price | ||||||||||||||||
| Existing stockholders | % | $ | % | $ | ||||||||||||||||
| New investors | $ | |||||||||||||||||||
| Total | 100.0 | % | 100.0 | % | $ | |||||||||||||||
If the underwriters exercise their option to purchase additional shares of our Common Stock in full, the percentage of shares of Common Stock held by existing stockholders will decrease to approximately % of the total number of shares of our Common Stock outstanding after this offering, and the number of shares held by new investors will increase to , or approximately % of the total number of shares of our Common Stock outstanding after this offering.
The foregoing tables and calculations are based on shares of our Common Stock outstanding as of December 31, 2020, and excludes:
| ● | shares of Common Stock issuable upon exercise of warrants to be issued to the representative of the underwriters as part of this offering at an exercise price of $ (assuming an initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus)). |
54
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of financial condition and operating results together with our financial statements and the related notes and other financial information included elsewhere in this prospectus. This discussion contains forward-looking statements that involve risks and uncertainties. As a result of many factors, such as those set forth in the section of the prospectus captioned “Risk Factors” and elsewhere in this prospectus, our actual results may differ materially from those anticipated in these forward-looking statements. For convenience of presentation some of the numbers have been rounded in the text below.
Overview
The Company was incorporated in the State of Delaware on May 12, 2020. The Company is engaged in psychiatric and neurological research regarding CNS disorders with the goal of translating this research into clinic-ready drugs.
The Company’s secondary operations focus on establishing anti-depression clinics across the UK and providing business support services to similar entities in the US, using trained pharmacists to administer intravenous infusions of ketamine. Pasithea has partnered with two successful clinics for immediate exposure in locations across Los Angeles, New York City and London.
The Company is located in Miami Beach, Florida, USA.
As of March 26, 2021, the Company had not commenced core operations. All activity for the period from May 12, 2020 (inception) through March 26, 2021 relates to the Company’s formation and raising funds through issuing shares of the Company’s Common Stock. The Company has selected December 31 as its fiscal year end.
The audited consolidated financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The consolidated financial statements include the consolidated financial statements of the Company and its wholly owned subsidiaries, Pasithea Therapeutics Limited (UK) and Pasithea Clinics Inc. All inter-company balances and transactions among the companies have been eliminated upon consolidation.
Impact of COVID-19 Pandemic
In March 2020, the World Health Organization characterized the outbreak of the novel strain of coronavirus, specifically identified as COVID-19, as a global pandemic. This has resulted in governments enacting emergency measures to combat the spread of the virus. These measures, which include the implementation of travel bans, self-imposed quarantine periods and social distancing, have caused material disruption to business, resulting in a global economic slowdown. Equity markets have experienced significant volatility and weakness and the governments and central banks have reacted with significant monetary and fiscal interventions designed to stabilize economic conditions.
The current challenging economic climate may lead to adverse changes in cash flows, working capital levels and/or debt balances, which may also have a direct impact on the Company’s operating results and financial position in the future. The ultimate duration and magnitude of the impact and the efficacy of government interventions on the economy and the financial effect on the Company is not known at this time. The extent of such impact will depend on future developments, which are highly uncertain and not in the Company’s control, including new information which may emerge concerning the spread and severity of COVID-19 and actions taken to address its impact, among others. The repercussions of this health crisis could have a material adverse effect on the Company’s business, financial condition, liquidity and operating results.
In response to COVID-19, the Company has implemented working practices to address potential impacts to its operations, employees and customers, and will take further measures in the future if and as required. At present, we do not believe there has been any appreciable impact on the Company specifically associated with COVID-19.
55
Emerging Growth Company Status
The Company is an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and it may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company which is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
RESULTS OF OPERATIONS
For the Period from Inception Through December 31, 2020
For the period from Inception through December 31, 2020, we incurred operating expenses of $40,984. The operating expenses were attributable to selling, general and administrative fees.
Net Loss
For the period from Inception through December 31, 2020, we incurred a net loss of $40,984.
Liquidity and Capital Resources
As of December 31, 2020, we had $247,958 in current assets and $6,603 in current liabilities. We had $243,650 in cash and cash equivalents and our accumulated deficit was $40,984.
Cash Flows:
| December 31, 2020 | ||||
| Cash Flows From Operating Activities | $ | (38,689 | ) | |
| Cash Flows From Financing Activities | 282,339 | |||
| Net decrease in cash and cash equivalents | $ | 243,650 | ||
Cash Flows From Operating Activities
We used $38,689 of cash in our operating activities. These are attributable to our net loss adjusted by changes in prepaid insurance of $4,308 and by changes in accounts payable and accrued liabilities of $6,603.
56
Cash Flows From Financing Activities
We received $282,339 from the issuance of Common Stock.
Off-Balance Sheet Arrangements
We did not have any off-balance sheet arrangements as defined in Item 303(a)(4)(ii) of Regulation S-K promulgated under the Exchange Act.
Contractual Obligations and Commitments
We did not have any contractual obligations.
Critical Accounting Policies
Use of Estimates
The preparation of financial statement in conformity with GAAP requires the Company’s management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statement and the reported amounts of revenues and expenses during the reporting period.
Making estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the financial statement, which management considered in formulating its estimate, could change in the near term due to one or more future confirming events. Accordingly, the actual results could differ significantly from those estimates.
Cash and cash equivalents
The Company considers all short-term investments with an original maturity of three months or less when purchased to be cash equivalents. As of December 31, 2020, we had no cash balances in bank deposit accounts that exceeded federally insured limits.
Income Taxes
The Company follows the asset and liability method of accounting for income taxes under ASC 740, “Income Taxes.” Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that included the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
ASC 740 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities. The Company recognizes accrued interest and penalties related to unrecognized tax benefits as income tax expense. There were no unrecognized tax benefits and no amounts accrued for interest and penalties as of December 31, 2020. The Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation from its position. The Company is subject to income tax examinations by major taxing authorities since inception.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist of a cash account in a financial institution, which, at times, may exceed the Federal Depository Insurance Coverage of $250,000. As of December 31, 2020, the Company has not experienced losses on this account and management believes the Company is not exposed to significant risks on such account.
57
Fair Value of Financial Instruments
The fair value of the Company’s assets and liabilities, which qualify as financial instruments under ASC 820, “Fair Value Measurements and Disclosures,” approximates the carrying amounts represented in the accompanying balance sheet, primarily due to their short-term nature.
Net Loss Per Share
Net loss per share is computed by dividing net loss by the weighted average number of common shares outstanding during the reporting period. Diluted earnings per share is computed similar to basic earnings per share, except the weighted average number of common shares outstanding are increased to include additional shares from the assumed exercise of share options, if dilutive. There are no outstanding dilutive or potentially dilutive instruments.
Recent Accounting Pronouncements
Management does not believe that any recently issued, but not yet effective, accounting pronouncements, if currently adopted, would have a material effect on the Company’s financial statement.
Going Concern and Management’s Liquidity Plans
As of December 31, 2020, the Company had $243,650 in its operating bank account, and working capital of $241,355. The Company’s liquidity needs up to December 31, 2020 had been satisfied through proceeds from the issuance of Common Stock.
The accompanying consolidated financial statements have been prepared on the basis that the Company will continue as a going concern, which assumes the realization of assets and the satisfaction of liabilities in the normal course of business. As of December 31, 2020, the Company has an accumulated deficit of $40,984 and has experienced losses from continuing operations. Based on the Company’s cash balance as of December 31, 2020, and projected cash needs for 2021, management estimates that it will need to increase sales revenue and/or raise additional capital to cover operating and capital requirements. Management will need to raise the additional funds through issuing additional shares of Common Stock or other equity securities or obtaining debt financing. Although management has been successful to date in raising necessary funding, there can be no assurance that there will be sales revenue or that any required future financing can be successfully completed on a timely basis, or on terms acceptable to the Company. Based on these circumstances, management has determined that these conditions raise substantial doubt about the Company’s ability to continue as a going concern.
Accordingly, the accompanying consolidated financial statements have been prepared in conformity with U.S. GAAP, which contemplates continuation of the Company as a going concern and the realization of assets and the satisfaction of liabilities in the normal course of business. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
58
Overview
We are a biotechnology company focused on the research and discovery of new and effective treatments for psychiatric and neurological disorders. Epidemiological data indicate neuropsychiatric disorders as being some of the most prevalent, devastating, and yet poorly treated illnesses. We believe that the current treatments for these disorders, such as depression, are inadequate and that conventional medicines have low success rates in long-term treatment. For example, current pharmacotherapies for MDD and BDep have a distinct lag of onset that can generate further distress and impairment in patients. Traditional psychiatric drugs can also cause serious side effects. Furthermore, the approval of psychotropic drugs with novel mechanisms of action has been rare in recent years. Our biotech operations will focus on developing drugs that target the pathophysiology underlying such disorders rather than symptomatic treatments, with the goal of developing new pharmacological agents that display significant advantages over conventional therapies with respect to efficacy and tolerability. We will particularly focus on the cross-talk between the immune system and brain disorders and how immune dysregulation affects CNS function. Our drug discovery efforts will focus on neuropsychiatric disorders that although phenotypically distinct are pathogenically related. We aim to focus on mechanism-based immune treatments for the treatment of these disorders.
Our secondary operations are focused on establishing anti-depression clinics across the United Kingdom and providing business support services to similar entities in the United States and using psychiatric assessment combined with physician/medical providers to administer intravenous infusions of ketamine. Operations will initially take place across the United States and the United Kingdom through partnerships with healthcare companies, including with Zen Healthcare and The IV Doc.
Ketamine was first introduced to the medical community as a surgical anesthetic more than 50 years ago. According to “Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review,” a 2015 article published by Therapeutic Advances in Chronic Disease, a peer-reviewed open access journal, and “Ketamine for major depression: New tool, new questions,” a 2019 article published on the Harvard Medical School’s website, as of the date of this prospectus, ketamine is gaining grounds as a promising treatment for some cases of major depression. It works differently than traditional antidepressants, which target the brain’s serotonin and noradrenalin systems. Ketamine blocks NMDA, a receptor in the brain that is activated by glutamate, a neurotransmitter. A single subanesthetic dose infusion of the NMDA receptor antagonist ketamine has been shown to have potentially rapid and potent antidepressant effects in treatment-resistant MDD as well as for the treatment of post-traumatic stress disorder. While not approved by the FDA or the MHRA to treat depression, and while recreational use remains prohibited, subject to appropriate caution and review, doctors and pharmacists prescribe ketamine for medical purposes, a practice endorsed by the American Psychiatric Association. Ketamine’s potential safety and effectiveness have been demonstrated in multiple research studies. As many as 70% of those who get ketamine infusions show a response, typically after the first session. If a person responds to ketamine, it may rapidly reduce suicidality and relieve other symptoms of depression.
As of March 26, 2021, the Company had not commenced core operations. All activity for the period from May 12, 2020 (inception) through March 26, 2021 relates to the Company’s formation and raising funds through issuing shares of the Company’s Common Stock. The Company has selected December 31 as its fiscal year end.
Our Strategy
Our core strategy is to become a leader in solving psychiatric and neurological disorders, one of the world’s biggest clinical problems, through research, development, and commercialization of novel CNS drugs. Key elements of our business strategy are as follows:
| ● | Research new drugs with different mechanism of action to conventional psychiatric and neurological drugs, targeting the pathophysiology underlying the disease, for the treatment of CNS disorders under the leadership of Professor Lawrence Steinman, a renowned neurologist and immunologist based at Stanford University, and Dr. Tiago Reis Marques, a psychiatrist and neuroscientist at Imperial College and King’s College London; |
59
| ● | Partner with reputable and successful healthcare companies and clinics to provide and support the intravenous administration of ketamine to treat treatment-resistant depression; | |
| ○ | Create a capital efficient revenue stream with significant client bases across the United States and the United Kingdom, including in Los Angeles, New York City, and London; and | |
| ○ | Create a diversified revenue stream by establishing and supporting clinics to provide greater visibility of revenue and EBITDA. |
Development Pipeline
Our current research plan, which is aimed at developing new molecular entities and/or novel biologic drugs in the 24 months following the closing of this offering, is as follows:
1. Selection of Candidates. We plan to select and develop three lead candidate compounds focused on the neurobiology of psychiatric and neurological disorders that can be developed into drugs and which have commercial potential as drug targets.
2. Hit to Lead Stage. Next, we plan to put the candidate compounds through a hit to lead stage, which is a stage in early drug discovery where small molecule hits from a high throughput screen are evaluated and undergo limited optimization to identify promising lead compounds. The candidate compounds will undergo chemistry characterization, compound metabolism, pharmacokinetics, in vitro pharmacology, in vivo pharmacology, and safety assays.
3. Disease Models. We plan to use preclinical models of psychiatric and neurological disorders, as the lead compounds are cleared. Our research will combine a conservative approach, under which lead compounds will be sought on a well-defined target, and a moonshot approach, under which completely novel mechanisms of action will be researched.
After 24 months, and after we develop one or more product candidates, subject to FDA and other similar regulatory approvals, we aim to begin one or more clinical trials.
About Our Indications
According to the National Institute of Mental Health, mental illnesses are common in the United States. Mental illnesses include many different conditions that vary in degree of severity, ranging from mild to moderate to severe. Two broad categories can be used to describe these conditions: AMI and SMI. AMI encompasses all recognized mental illnesses, whereas SMI is a smaller and more severe subset of AMI.
In 2019, there were an estimated 51.5 million adults aged 18 or older in the United States with AMI. Among the 51.5 million adults with AMI, 23.0 million (44.8%) received mental health services in the past year. In 2019, there were an estimated 13.1 million adults aged 18 or older in the United States with SMI, which represented 5.2% of all U.S. adults. Out of the 13.1 million adults with SMI, 8.6 million (65.5%) received mental health treatment in the past year.
According to the Mayo Clinic, treatment for mental illness largely depends on the type of mental illness and its severity. Currently, treatment can include psychiatric medication (such as anti-depressants, anti-anxiety medications, mood stabilizers, and antipsychotic drugs), psychotherapy, brain-stimulation treatments, hospitalization, substance misuse treatment, or any combination of the foregoing.
Clinical Services
Our secondary operations are focused on establishing anti-depression clinics across the United Kingdom and providing business support services to similar entities in the United States, using psychiatric assessment combined with physician/medical providers to provide private intravenous infusions of ketamine to treat treatment-resistant depression through partnerships with healthcare companies including with Zen Healthcare and The IV Doc.
60
United Kingdom. Pasithea’s United Kingdom branch has already partnered with Zen Healthcare, a general practice group with three locations: Marylebone, Knightsbridge, and Holborn. Zen Healthcare has been operating for five years and has approximately 30,000 patients. Its practices give us immediate exposure in the United Kingdom. Other advantages including gaining access to an existing management structure and qualified general practitioners, pharmacists, therapists, and psychotherapists. In the future, we plan to open independent clinics in London and other top regional cities in the United Kingdom.
In the UK, Pasithea Therapeutics Corp. has established a wholly owned subsidiary organized under United Kingdom Law to provide psychotherapy and to administer IV ketamine in clinics. Under the laws of the UK, this entity may directly own and operate clinics, employ physicians, and provide management services to clinics and providers. In order to do so, the UK entity must obtain approvals from the following agencies: MHRA, CQC, GMC and the GPC.
Specifically, in the UK, Pasithea will be responsible for obligations such as maintaining a CQC license, marketing ketamine and other treatments, booking and taking payments from patients, providing licensed and qualified staff and all pharmaceuticals and equipment necessary for the assessment of patients and provision of the treatments, assessing patients, and administering treatments. At the present, Pasithea has partnered with Purecare Limited and Portman Health Ltd that own Zen clinics to treat patients, including providing psychiatric consultations, and that have pharmacies that will procure, handle, and administer ketamine in treatment rooms, providing all pharmaceuticals and equipment necessary for the assessment of patients and the provision of the treatments.
In the UK, ketamine is a Schedule II controlled substance under the Misuse of Drugs Regulations 2010 and is controlled with regard to synthesis, storage and distribution under the Misuse of Drugs Act 1971 as amended. Possession of ketamine requires Home Office licensing and may only be stored on premises complying with professional strictures of the GPC. As a controlled substance, ketamine requires production and supply from a manufacturer possessing MHRA manufacturing authorization which ensures the production of GMP quality ketamine. Additionally, like in the US, because IV ketamine has not yet been granted marketing authorization for the psychotherapy indication in the UK, it must be regarded as an unlicensed medicine that is being used off label without its authorized indications for anesthesia and/or chronic pain. The GMC code of good practice allows a physician to prescribe an unlicensed medicine under his own responsibility.
Specifically, in the UK, our operation process are as follows:
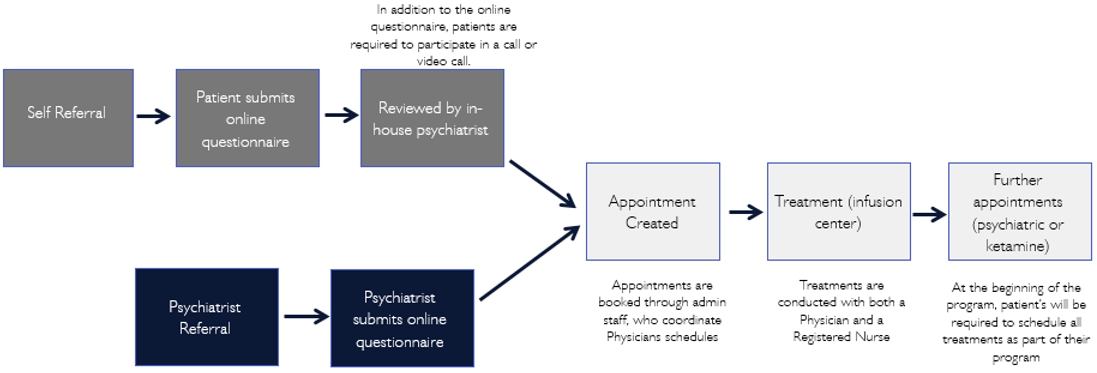
United States (including New York and California). In the United States, Pasithea has partnered with The IV Doc. The IV Doc itself and through clinical affiliates has treated over 50,000 patients over the past seven years. During that period, The IV Doc has established relationships with over 800 clinicians. Through these efforts, The IV Doc has developed a national reputation for the provision of in-home infusion services, testing, and outpatient medical care. Pasithea’s operations in New York and Los Angeles can be efficiently expanded to other locations utilizing The IV Doc patient service delivery model, including The IV Doc software and technology and clinical services management resources.
61
In the United States, the FDA, the DEA and state agencies regulate the use, maintenance and distribution of ketamine. At the federal level, FDA has approved ketamine for use as an anesthetic but not for subanesthetic intravenous administration for psychotherapy. However, in general, physicians may prescribe FDA-approved drugs for conditions other than what the drugs have been explicitly approved for (off-label use). Once a drug such as ketamine is approved for any use, physicians may prescribe those drugs for off-label uses consistent with applicable state medical practice requirements (see below). Thus, no new or additional approvals are required from the FDA for the off-label use of ketamine for proper medical use like in this instance. The DEA, under the federal Controlled Substance Act, oversees the maintenance and distribution of all controlled substances, including ketamine. Depending on the specific clinical protocols and standards established by the independent professional services company and the contracted or employed physicians prescribing and administering ketamine, the entity and/or the contracted or employed physicians will be required to comply with all DEA requirements.
In the Initial States, Pasithea is in the process of establishing management agreements with independent professional services companies that are in the process of formation that will be organized and established under the laws of the Initial States, including all laws related to the corporate practice of medicine, fee-splitting, licensure, and fraud and abuse laws. The independent professional services companies, through their physicians and nurses, will perform the clinical operations related to the business model. Individual clinicians, including psychiatrists, anesthesiologists, and nurses, all licensed and qualified to provide the clinical services required, will contract with the independent professional services companies to provide the services (see below). The independent professional services companies will be owned by a separate shareholder or shareholders from Pasithea. Through its management agreement, Pasithea, in conjunction with The IV Doc, will provide all of the non-clinical management services necessary for the professional services companies to operate, including administrative services, information technology services and marketing services, online advertising, and other channels. Pasithea has entered into a subcontract agreement with The IV Doc to provide for the subcontracting of certain administrative, information technology, and billing services provided by The IV Doc to us.
In New York, the independent New York professional services company will be organized under Section 1203 of the New York Limited Liability Company Law and in compliance with Article 15 of the New York Business Corporation Law. This entity filed for certification with the New York Department of Education, which application is consistent with the requirements of the regulations of the Commissioner of Education, §59.10. Upon approval by the New York Department of Education, this entity will then file for corporate approval by the New York Department of State by submitting its articles of organization. This entity will also apply for an EIN with the IRS. At that point, this independent entity will be ready to perform the contemplated clinical services directly and through its employed or contracted physicians.
In New York, licensed New York psychiatrists will perform the initial diagnostic services to patients. These psychiatrists will be required to be licensed and otherwise qualified to perform these services under NY Education Law, Article 131, §6520. Thereafter, these patients will be evaluated and, where appropriate, administered IV ketamine by licensed anesthesiologists. These anesthesiologists will be licensed under NY Education Law, Article 131, §6520. These anesthesiologists will be assisted by registered nurses, including CRNAs, who will be licensed under NY Education Law Article 139, §6900, et seq. The model clinical services agreements developed by the independent professional services company will reflect these requirements. All clinicians will be required to possess and maintain such New York licenses during the course of their employment or contractual obligations.
In California, the independent California professional services company will be organized under California Corporation Code § 13400, et seq. The California professional services company will file articles of incorporation with the California Secretary of State. This entity will also apply for an EIN with the IRS. At that point, this independent entity will be ready to perform the contemplated clinical services directly and through its employed or contracted physicians.
In California, licensed psychiatrists will perform the initial diagnostic services to patients. These psychiatrists will be required to be licensed and otherwise qualified to perform these services under California Business and Professions Code §2000, et seq. and California Corporations Code §13400, et seq. Thereafter, these patients will be evaluated and, where appropriate, administered IV ketamine by licensed anesthesiologists under California Business and Professions Code §2000. These anesthesiologists will be assisted by registered nurses and CRNAs. The registered nurses will be licensed under California Business and Professions Code §2700, et seq. CRNAs will be licensed under 16 CCR § 1409. All clinicians will be required to possess and maintain such California licenses during the course of their employment or contractual obligations.
62
The process of ketamine infusion treatment entails first conducting a psychiatric assessment of the patient to determine the appropriateness of the treatment, then administering infusion treatment, and finally conducting psychiatric follow-ups. Specifically, in the United States, our operation process are as follows (in the United States, Pasithea will only provide business support services to other entities providing the relevant medical services):
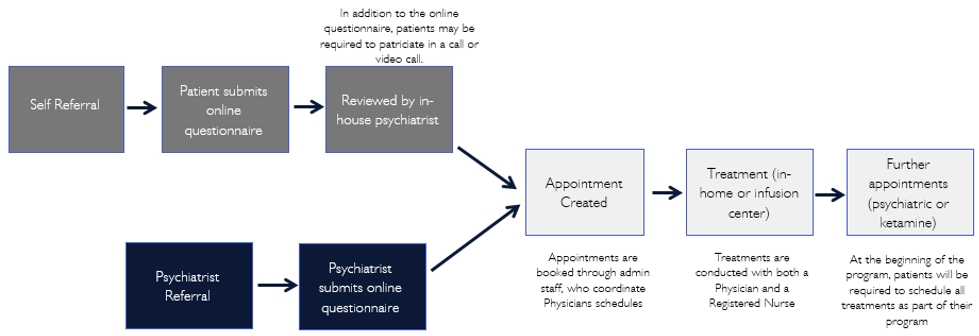
Our Team
We are founded and led by a best-in-class management team:
| ● | Professor Lawrence Steinman, Executive Chairman and Co-Founder. Professor Steinman has served on our board of directors since August 2020. Prior to joining Pasithea, he served on the Board of Directors of Centocor from 1989 to 1998, the Board of Directors of Neurocine Biosciences from 1997 to 2005, the Board of Directors of Atreca from 2010 to 2019, the Board of Directors of BioAtla from 2016 to the present, and the Board of Directors of Tolerion from 2013 to the present. He is currently the George A. Zimmermann Endowed Chair in the Neurology Department at Stanford University and previously served as the Chair of the Interdepartmental Program in Immunology at Stanford University Medical School from 2003 to 2011. He is a member of the National Academy of Medicine and the National Academy of Sciences. He also founded the Steinman Laboratory at Stanford University, which is dedicated to understanding the pathogenesis of autoimmune diseases, particularly multiple sclerosis and neuromyelitis optica. He received the Frederic Sasse Award from the Free University of Berlin in 1994, the Sen. Jacob Javits Award from the U.S. Congress in 1988 and 2002, the John Dystel Prize in 2004 from the National MS Society in the U.S., the Charcot Prize for Lifetime Achievement in Multiple Sclerosis Research in 2011 from the International Federation of MS Societies and the Anthony Cerami Award in Translational Medicine by the Feinstein Institute of Molecular Medicine in 2015. He also received an honorary Ph.D. at the Hasselt University in 2008. He received his BA (physics) from Dartmouth College in 1968 and his MD from Harvard University in 1973. He also completed a fellowship in chemical immunology at the Weizmann Institute (1974 – 1977) and was an intern and resident at Stanford University Medical School. | |
| ● | Dr. Tiago Reis Marques, Chief Executive Officer and Director. Dr. Marques has served on our board of directors and as Chief Executive Officer since August 2020. He is a senior clinical fellow at Imperial College London and a lecturer at the IoPPN, King’s College London. IoPPN is ranked second in the world for psychology and psychiatry by US News and Best Global Universities, and is home to one of the world’s largest centers for neuroscience research. Dr. Marques is also a psychiatrist at Maudsley Hospital. His research focuses on topics including the mechanism of action of psychiatric medication and novel treatment targets. During his career, he has obtained multiple awards for his research. Dr. Marques is an author or co-author of more than 100 scientific publications in peer-reviewed journals in psychiatry and neuroscience, has co-authored international treatment guidelines and written book chapters, including in the leading book in the field, “Neurobiology of Mental Illness.” |
| ● | Stanley M. Gloss, Chief Financial Officer. Mr. Gloss has served as our Chief Financial Officer since April 2021. He has been self-employed for the past year doing financial consulting in the areas of accounting and financial reporting. From 2017 to 2020, Mr. Gloss was Controller at Ace Universe, establishing and maintaining the budgets and financial reporting systems and sourcing and maintaining the company insurance. From 2009 to 2016, Mr. Gloss was Controller and Vice President of Finance of Wizard World Inc., where he established and maintained the budgets and financial reporting systems, sourced and maintained the company contracts and insurance, and coordinated public filings. He received his Bachelor of Science in Accounting from Fairfield University. |
63
| ● | Dr. Yassine Bendiabdallah, Chief Operating Officer, Head of UK Clinics and Director. Dr. Bendiabdallah has served on our board of directors and as Chief Operating Officer since March 2021. He also co-founded Pasithea Therapeutics Corp. and is currently Head of UK Clinics. Dr. Bendiabdallah is an expert in functional medicine and bio-identical hormone therapy. He completed a Masters in Pharmacy at King’s College London in 2006. He was then awarded a PhD scholarship within Cancer Research UK group at University Colleges London which was completed with honours in 2010. He then went on to work for a number of pharmaceutical companies and held research position at University College London. He has been involved in several startups including HelloDr (HelloDr Ltd, Proximal Health Ltd) an online tech in healthcare, Androgenix Pharmaceuticals Ltd, and Purecare Ltd (Zen Healthcare) which he is the co-founder and current managing director. Zen Healthcare now comprises several clinics and pharmacies in the UK. He holds a number of scientific publications in peer-reviewed literature the anticancer research industry. Dr. Bendiabdallah has also attended and presented at several seminars and conferences globally. His current clinical expertise includes age reversal therapies, functional approaches to medicines and intravenous micronutrient therapies. | |
| ● | Simon Dumesnil, Director. Mr. Dumesnil has served on our board of directors since April 2021. He is currently a Managing Partner and Director of Dunraven Capital Partners Limited, an investment management advisory company incorporated in the UK whose investments are predominately in Eastern European corporate distressed credits and structured products. From 2013 to 2018, Mr. Dumesnil was Managing Director and Head of Structured Financing Group Americas of UBS Securities LLC, where he was responsible for the structured financing trading book in the USA and LATAM and managed a book of financing positions across fixed income products (corporate syndicated and middle-market loans, corporate bonds, real estate loans, CMBS/RMBS/CLO/ABS, LATAM Sovereign). From 2010 to 2013, he was Managing Director and Co-Head Private-Side Structuring Group EMEA of UBS AG., where he was responsible for arranging structured solution transactions and acquisitions for FIG and Special Situation Group (SSG) and also co-headed the illiquid financing business. From 2009 to 2010, Mr. Dumesnil was the Chief Investment Officer Bluestone Capital Management and responsible for investments in distressed assets across Europe. From 2008 to 2009, Mr. Dumesnil was Director of Lehman Brother Holding Inc. and responsible for restructuring and unwinding Lehman Brothers Special Financing Inc. derivative book post-bankruptcy. From 2003 to 2008, Mr. Dumesnil was Director of Lehman Brothers International (Europe). Throughout his career at Dunraven Capital Management, UBS Securities, UBS AG, Bluestone Capital Management and Lehman Brothers, Mr. Dumesnil advised and underwritten corporate risk related to companies across industries or jurisdictions. He has an in-depth knowledge on corporate restructuring and capital structure optimization for companies across their business life cycle. His experience as Chief Investment Officer during the launch and growth phases of a financial services and technology company represents valuable insights for our Company. Mr. Dumesnil attended Cass Business School, where he received his Master of Science in Banking and International Finance and École des Hautes-Études-Commerciales HEC, where he received his Bachelor in Business and Administration, Finance. | |
Other Partnerships
In addition to our clinic partnerships described above, we anticipate partnering both with contract research organizations and educational institutions to help develop our product candidates and, eventually, to support our clinical trials.
Manufacturing
We anticipate devoting significant resources to process development and manufacturing to optimize process robustness and success rates in developing potential product candidates with financially viable per-unit manufacturing costs and enable us to quickly achieve regional and global scale production upon regulatory approval for our future product candidates.
Financial Overview
We have experienced losses since inception and, at December 31, 2020, had an accumulated deficit of approximately $40,984. We expect to incur additional losses in the future and expect cumulative losses to increase. Since May 2020, we have received approximately $1.47 million in equity financing in connection with which we issued 8,307,327 shares of Common Stock to approximately 46 accredited investors through a series of financings conducted pursuant to the Rule 506(b) Regulation D “safe harbor” for the private offering exemption of Section 4(a)(2) of the Securities Act completed in January 2021.
64
Competition
The pharmaceutical market for the treatment of major depressive disorder includes selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors and atypical antipsychotics. A number of these marketed antidepressants will be generic, and would be key competitors to our ketamine drug candidate. These products include Janssen Pharmaceuticals, Inc.’s Spravato (esketamine), Forest Laboratory’s Lexapro/Cipralex (escitalopram) and Viibryd (vilazodone), Pfizer, Inc.’s Zoloft (sertraline), Effexor (venlafaxine) and Pristiq (desvenlafaxine), GlaxoSmithKline plc’s Paxil/Seroxat (paroxetine), Eli Lilly and Company’s Prozac (fluoxetine) and Cymbalta (duloxetine), AstraZeneca plc’s Seroquel (quetiapine) and Bristol-Myers Squibb Company’s Abilify (aripiprazole), among others.
We anticipate that competition in our industry will increase. In addition, the health care industry is characterized by rapid technological change, resulting in new product introductions and other technological advancements. Our competitors may develop and market products that render future product candidates, or any products manufactured or marketed by us, non-competitive or otherwise obsolete.
Competition in Clinic Model
The following clinics use psychiatric assessment combined with physician/medical providers to administer intravenous infusions of ketamine.
| ● | Ketamine Clinics LA | |
| ● | California Ketamine Clinics | |
| ● | Ketamine Healing Clinic of LA | |
| ● | TMS & Brain Health | |
| ● | NY Ketamine Infusions | |
| ● | Field Trip Health | |
| ● | MindBody Therapeutics | |
Intellectual Property
We currently do not hold any intellectual property, but intend to develop product candidates that may be the subject of future patent applications.
License Agreements and Strategic Collaborations
Zen Clinics
During the year ended December 31, 2020, the Company entered into a Collaboration Agreement (the “Zen Knightsbridge and Holborn Collaboration Agreement”) with Purecare Limited (“Purecare”), a company that operates a health clinic known as Zen Knightsbridge Clinic (the “Zen Knightsbridge Clinic”), whereby both parties have agreed to collaborate on the provision of treatments at Purecare’s London based clinic. The Company has agreed to apply and maintain necessary licenses, market the treatments, and develop and maintain a website for online booking and payments of treatments. Purecare has agreed to provide consulting and treatment rooms at its clinics, as well as providing all pharmaceuticals and equipment necessary for the assessment of patients and provisions of the treatments. All resulting revenue from such treatments shall be allocated 30% to the Company and 70% to Purecare.
65
Under the Zen Knightsbridge and Holborn Collaboration Agreement, we are responsible for obligations such as maintaining a CQC license, marketing ketamine and other treatments, booking and taking payments from patients, providing licensed and qualified staff and all pharmaceuticals and equipment necessary for the assessment of patients and provision of the treatments, assessing patients, and administering treatments at the Zen Knightsbridge Clinic. Purecare is responsible for providing consulting and treatment rooms at the Zen Knightsbridge Clinic and providing all pharmaceuticals and equipment necessary for the assessment of patients and the provision of the treatments. All profit, after deduction of expenses, shall be split 70/30, with Purecare receiving 70% and us receiving 30%. The initial term of the Zen Knightsbridge and Holborn Collaboration Agreement commenced during the year ended December 31, 2020 and will continue in effect for five years. After the initial term, the Zen Knightsbridge and Holborn Collaboration Agreement will automatically extend for five years unless otherwise terminated. We have the right to terminate the Zen Knightsbridge and Holborn Collaboration Agreement at any time by giving Purecare at least six months’ prior written notice.
During the year ended December 31, 2020, the Company entered into a Collaboration Agreement (the “Zen Baker Collaboration Agreement”) with Portman Health Ltd (“Portman”), a company that operates a health clinic known as Zen Baker Street Clinic (the “Zen Baker Clinic”). Under the Zen Baker Collaboration Agreement, we are responsible for obligations such as maintaining a CQC license, marketing ketamine and other treatments, booking and taking payments from patients, providing licensed and qualified staff and all pharmaceuticals and equipment necessary for the assessment of patients and provision of the treatments, assessing patients, and administering treatments at the Zen Baker Clinic. Portman is responsible for providing consulting and treatment rooms at the Zen Baker Clinic and providing all pharmaceuticals and equipment necessary for the assessment of patients and the provision of the treatments. All profit, after deduction of expenses, shall be split 70/30, with Portman receiving 70% and us receiving 30%. The initial term of the Zen Baker Collaboration Agreement commenced during the year ended December 31, 2020 and will continue in effect for five years. After the initial term, the Zen Baker Collaboration Agreement will automatically extend for five years unless otherwise terminated. We have the right to terminate the Zen Baker Collaboration Agreement at any time by giving Portman at least six months’ prior written notice.
Government Regulation and Drug Approval
Governmental Regulations
Government authorities in the United States (including federal, state and local authorities) and in other countries, extensively regulate, among other things, the manufacturing, research and clinical development, marketing, labeling and packaging, storage, distribution, post-approval monitoring and reporting, advertising and promotion, pricing and export and import of pharmaceutical products, such as our future product candidates. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Moreover, failure to comply with applicable regulatory requirements may result in, among other things, warning letters, clinical holds, civil or criminal penalties, recall or seizure of products, injunction, disbarment, partial or total suspension of production or withdrawal of the product from the market. Any agency or judicial enforcement action could have a material adverse effect on us.
U.S. Government Regulation
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act (“FDCA”) and its implementing regulations. Drugs are also subject to other federal, state and local statutes and regulations. The FDA’s Center for Drug Evaluation and Research would have primary jurisdiction over the premarket development, review and approval of our future product candidates. Accordingly, we have and plan to continue to investigate our products through the IND framework and seek approval through the NDA pathway. The process required by the FDA before our product candidates may be marketed in the United States generally involves the following:
| ● | submission to the FDA of an IND which must become effective before human clinical trials may begin and must be updated annually; |
| ● | completion of extensive preclinical laboratory tests and preclinical animal studies, all performed in accordance with the FDA’s Good Laboratory Practice regulations; |
| ● | performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the product candidate for each proposed indication in accordance with good clinical practices (“GCPs”); |
66
| ● | submission to the FDA of an NDA after completion of all pivotal clinical trials; |
| ● | a determination by the FDA within 60 days of its receipt of an NDA to file the NDA for review; |
| ● | satisfactory completion of an FDA pre-approval inspection of the manufacturing facilities at which the active pharmaceutical ingredient (“API”), and finished drug product are produced and tested to assess compliance with good manufacturing Practices (“cGMP”) regulations; and |
| ● | FDA review and approval of an NDA prior to any commercial marketing or sale of the drug in the United States. |
An IND is a request for authorization from the FDA to administer an investigational drug product to humans. The central focus of an IND submission is on the general investigational plan and the protocol(s) for human studies. The IND also includes results of animal studies or other human studies, as appropriate, as well as manufacturing information, analytical data and any available clinical data or literature to support the use of the investigational new drug. An IND must become effective before human clinical trials may begin. An IND will automatically become effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to the proposed clinical trials. In such a case, the IND may be placed on clinical hold and the IND sponsor and the FDA must resolve any outstanding concerns or questions before clinical trials can begin. Accordingly, submission of an IND may or may not result in the FDA allowing clinical trials to commence.
Clinical trials involve the administration of the investigational drug to human subjects under the supervision of qualified investigators in accordance with GCPs, which include the requirement that all research subjects provide their informed consent for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety, and the efficacy criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. Additionally, approval must also be obtained from each clinical trial site’s institutional review board (“IRB”) before the trials may be initiated, and the IRB must monitor the study until completed. There are also requirements governing the reporting of ongoing clinical trials and clinical trial results to public registries.
The clinical investigation of a drug is generally divided into three phases. Although the phases are usually conducted sequentially, they may overlap or be combined. The three phases of an investigation are as follows:
| ● | Phase I. Phase I includes the initial introduction of an investigational new drug into humans. Phase I clinical trials are typically closely monitored and may be conducted in patients with the target disease or condition or in healthy volunteers. These studies are designed to evaluate the safety, dosage tolerance, metabolism and pharmacologic actions of the investigational drug in humans, the side effects associated with increasing doses, and if possible, to gain early evidence on effectiveness. During Phase I clinical trials, sufficient information about the investigational drug’s pharmacokinetics and pharmacological effects may be obtained to permit the design of well-controlled and scientifically valid Phase II clinical trials. The total number of participants included in Phase I clinical trials varies, but is generally in the range of 20 to 80. |
| ● | Phase II. Phase II includes controlled clinical trials conducted to preliminarily or further evaluate the effectiveness of the investigational drug for a particular indication(s) in patients with the disease or condition under study, to determine dosage tolerance and optimal dosage, and to identify possible adverse side effects and safety risks associated with the drug. Phase II clinical trials are typically well-controlled, closely monitored, and conducted in a limited patient population, usually involving no more than several hundred participants. |
| ● | Phase III. Phase III clinical trials are generally controlled clinical trials conducted in an expanded patient population generally at geographically dispersed clinical trial sites. They are performed after preliminary evidence suggesting effectiveness of the drug has been obtained, and are intended to further evaluate dosage, clinical effectiveness and safety, to establish the overall benefit-risk relationship of the investigational drug product, and to provide an adequate basis for product approval. Phase III clinical trials usually involve several hundred to several thousand participants. |
67
A pivotal study is a clinical study which adequately meets regulatory agency requirements for the evaluation of a drug candidate’s efficacy and safety such that it can be used to justify the approval of the product. Generally, pivotal studies are also Phase III studies but may be Phase II studies if the trial design provides a well-controlled and reliable assessment of clinical benefit, particularly in situations where there is an unmet medical need.
The FDA, the IRB or the clinical trial sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk. Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group provides authorization for whether or not a trial may move forward at designated check points based on access to certain data from the study. We may also suspend or terminate a clinical trial based on evolving business objectives and/or competitive climate.
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, detailed investigational drug product information is submitted to the FDA in the form of an NDA requesting approval to market the product for one or more indications. The application includes all relevant data available from pertinent preclinical and clinical trials, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product’s chemistry, manufacturing, controls and proposed labeling, among other things. Data can come from company-sponsored clinical trials intended to test the safety and effectiveness of a use of a product, or from a number of alternative sources, including studies initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and effectiveness of the investigational drug product to the satisfaction of the FDA.
Once the NDA submission has been accepted for filing, within 60 days following submission, the FDA’s goal is to review applications for new molecular entities within ten months of the filing date or, if the application relates to a serious or life-threatening indication and demonstrates the potential to provide a significant improvement in safety or effectiveness over currently marketed therapies, six months from the filing date. The review process is often significantly extended by FDA requests for additional information or clarification. The FDA may refer the application to an advisory committee for review, evaluation and recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it typically follows such recommendations.
After the FDA evaluates the NDA and conducts inspections of manufacturing facilities where the drug product and/or its active pharmaceutical ingredient will be produced, it may issue an approval letter or a complete response letter. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. A complete response letter indicates that the review cycle of the application is complete and the application is not ready for approval. A complete response letter may require additional clinical data and/or an additional pivotal Phase III clinical trial(s), and/or other significant, expensive and time-consuming requirements related to clinical trials, preclinical studies or manufacturing. Even if such additional information is submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. The FDA could also approve the NDA with a risk evaluation and mitigation strategy to mitigate risks, which could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. The FDA also may condition approval on, among other things, changes to proposed labeling, development of adequate controls and specifications, or a commitment to conduct one or more post-market studies or clinical trials. Such post-market testing may include Phase IV clinical trials and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization. Regulatory approval of oncology products often requires that patients in clinical trials be followed for long periods to determine the overall survival benefit of the drug.
After regulatory approval of a drug product is obtained, manufacturers are required to comply with a number of post-approval requirements. The holder of an approved NDA must report, among other things, certain adverse reactions and production problems to the FDA, to provide updated safety and efficacy information, and to comply with requirements concerning advertising and promotional labeling for the approved product. Also, quality control and manufacturing procedures must continue to conform to cGMP after approval to ensure and preserve the long-term stability of the drug product. The FDA periodically inspects manufacturing facilities to assess compliance with cGMP, which imposes extensive procedural, substantive and record keeping requirements. In addition, changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon us and any third-party manufacturers that we may decide to use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance.
68
We expect to rely on third parties for the production of clinical and commercial quantities of our future product candidates. Future FDA and state inspections may identify compliance issues at our facilities or at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct. In addition, discovery of previously unknown problems with a product or the failure to comply with applicable requirements may result in restrictions on a product, manufacturer or holder of an approved NDA, including withdrawal or recall of the product from the market or other voluntary, FDA-initiated or judicial action that could delay or prohibit further marketing. Newly discovered or developed safety or effectiveness data may require changes to a product’s approved labeling, including the addition of new warnings and contraindications, and also may require the implementation of other risk management measures. Also, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our products under development.
Expedited Development and Review Programs for Drugs
The FDA maintains several programs intended to facilitate and expedite development and review of new drugs to address unmet medical needs in the treatment of serious or life-threatening diseases or conditions. These programs include Fast Track designation, Breakthrough Therapy designation, Priority Review and Accelerated Approval, and the purpose of these programs is to either expedite the development or review of important new drugs to get them to patients more quickly than standard FDA review timelines typically permit.
A drug is eligible for Fast Track designation if it is intended to treat a serious or life-threatening disease or condition and demonstrates the potential to address unmet medical needs for such disease or condition. Fast Track designation provides increased opportunities for sponsor interactions with the FDA during preclinical and clinical development, in addition to the potential for rolling review once a marketing application is filed. Rolling review means that the agency may review portions of the marketing application before the sponsor submits the complete application. In addition, a drug may be eligible for Breakthrough Therapy designation if it is intended to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Breakthrough Therapy designation provides all the features of Fast Track designation in addition to intensive guidance on an efficient drug development program, and FDA organizational commitment to expedited development, including involvement of senior managers and experienced review staff in a cross-disciplinary review, where appropriate.
Any product submitted to the FDA for approval, including a product with Fast Track or Breakthrough Therapy designation, may also be eligible for additional FDA programs intended to expedite the review and approval process, including Priority Review designation and Accelerated Approval. A product is eligible for Priority Review designation, once an NDA or a biologics license application, or BLA, is submitted, if the drug that is the subject of the marketing application has the potential to provide a significant improvement in safety or effectiveness in the treatment, diagnosis or prevention of a serious disease or condition. Under priority review, the FDA’s goal date to take action on the marketing application is six months compared to ten months for a standard review. Products are eligible for Accelerated Approval if they can be shown to have an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or an effect on an intermediate clinical endpoint that can be measured earlier than an effect on irreversible morbidity or mortality, which is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments.
Accelerated Approval is usually contingent on a sponsor’s agreement to conduct additional post-approval studies to verify and describe the product’s clinical benefit. The FDA may withdraw approval of a drug or an indication approved under Accelerated Approval if, for example, the confirmatory trial fails to verify the predicted clinical benefit of the product. In addition, the FDA generally requires, as a condition for Accelerated Approval, that all advertising and promotional materials intended for dissemination or publication within 120 days of marketing approval be submitted to the agency for review during the pre-approval review period. After the 120-day period has passed, all advertising and promotional materials must be submitted at least 30 days prior to the intended time of initial dissemination or publication.
Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or the time period for FDA review or approval may not be shortened. Furthermore, Fast Track designation, Breakthrough Therapy designation, Priority Review and Accelerated Approval do not change the scientific or medical standards for approval or the quality of evidence necessary to support approval, though they may expedite the development or review process.
69
Controlled Substances
The federal Controlled Substances Act of 1970, or CSA, and its implementing regulations establish a “closed system” of regulations for controlled substances. The CSA imposes registration, security, recordkeeping and reporting, storage, manufacturing, distribution, importation and other requirements under the oversight of the DEA. The DEA is the federal agency responsible for regulating controlled substances, and requires those individuals or entities that manufacture, import, export, distribute, research, or dispense controlled substances to comply with the regulatory requirements in order to prevent the diversion of controlled substances to illicit channels of commerce.
The DEA categorizes controlled substances into one of five schedules — Schedule I, II, III, IV or V — with varying qualifications for listing in each schedule. Schedule I substances by definition have a high potential for abuse, have no currently accepted medical use in treatment in the United States and lack accepted safety for use under medical supervision. Pharmaceutical products having a currently accepted medical use that are otherwise approved for marketing may be listed as Schedule II, III, IV or V substances, with Schedule II substances presenting the highest potential for abuse and physical or psychological dependence, and Schedule V substances presenting the lowest relative potential for abuse and dependence. COMP360, if approved in the United States, will require scheduling by the DEA before it can be marketed.
To conduct clinical trials with controlled substances in the United States prior to approval, each of the research sites must submit a research protocol to the DEA and obtain and maintain a DEA researcher registration that will allow those sites to handle and dispense the products and to obtain the product from a supplier. If the DEA delays or denies the grant of a research registration to one or more research sites, the clinical trial could be significantly delayed, and the clinical trial sites could be lost. The supplier for the clinical trials must also obtain a Schedule I registration.
If any proposed products developed receive FDA approval, the DEA will make a scheduling determination and place it in a schedule other than Schedule I in order for it to be prescribed to patients in the United States. Consequently, its manufacture, importation, exportation, domestic distribution, storage, sale and legitimate use may be subject to a significant degree of regulation by the DEA. Our failure to comply with these regulations could result in the loss of our DEA registration, civil penalties or criminal prosecution. In addition, the scheduling process may take one or more years, thereby delaying the launch of any product in the United States. Furthermore, if the FDA, DEA, or any foreign regulatory authority determines that any product may have potential for abuse, it may require us to generate more clinical or other data than we currently anticipate to establish whether or to what extent the substance has an abuse potential, which could increase the cost and/or delay the launch of any proposed product.
Facilities that manufacture, distribute, import or export any controlled substance must register annually with the DEA. The DEA registration is specific to the particular location, activity(ies) and controlled substance schedule(s).
The DEA inspects all manufacturing facilities to review security, recordkeeping, reporting and handling prior to issuing a controlled substance registration. The specific security requirements vary by the type of business activity and the schedule and quantity of controlled substances handled. The most stringent requirements apply to manufacturers of Schedule I and Schedule II substances. Required security measures commonly include background checks on employees and physical control of controlled substances through storage in approved vaults, safes and cages, and through use of alarm systems and surveillance cameras. Once registered, manufacturing facilities must maintain records documenting the manufacture, receipt and distribution of all controlled substances. Manufacturers must submit periodic reports to the DEA of the distribution of Schedule I and II controlled substances, Schedule III narcotic substances, and other designated substances. Registrants must also report any controlled substance thefts or significant losses, and must obtain authorization to destroy or dispose of controlled substances. Imports of Schedule I and II controlled substances for commercial purposes are generally restricted to substances not already available from a domestic supplier or where there is not adequate competition among domestic suppliers. In addition to an importer or exporter registration, importers and exporters must obtain a permit for every import or export of a Schedule I and II substance or Schedule III, IV and V narcotic, and submit import or export declarations for Schedule III, IV and V non-narcotics. In some cases, Schedule III non-narcotic substances may be subject to the import/export permit requirement, if necessary, to ensure that the United States complies with its obligations under international drug control treaties.
For drugs manufactured in the United States, the DEA establishes annually an aggregate quota for the amount of substances within Schedules I and II that may be manufactured or produced in the United States based on the DEA’s estimate of the quantity needed to meet legitimate medical, scientific, research and industrial needs. The quotas apply equally to the manufacturing of the active pharmaceutical ingredient and production of dosage forms. The DEA may adjust aggregate production quotas a few times per year, and individual manufacturing or procurement quotas from time to time during the year, although the DEA has substantial discretion in whether or not to make such adjustments for individual companies.
70
The DEA, and some states, also conduct periodic inspections of registered establishments that handle controlled substances. Facilities that conduct research, manufacture, store, distribute, import or export controlled substances must be registered to perform these activities and have the security, control and inventory mechanisms required by the DEA to prevent drug loss and diversion. Failure to maintain compliance, particularly non-compliance resulting in loss or diversion, can result in regulatory action that could have a material adverse effect on our business, results of operations, financial condition and prospects. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to revoke those registrations. In certain circumstances, violations could lead to criminal proceedings.
The states also maintain separate controlled substance laws and regulations, including licensing, recordkeeping, security, distribution, and dispensing requirements. State authorities, including boards of pharmacy, regulate use of controlled substances in each state. Failure to maintain compliance with applicable requirements, particularly as manifested in the loss or diversion of controlled substances, can result in enforcement action that could have a material adverse effect on our business, operations and financial condition. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to revoke those registrations. In certain circumstances, violations could lead to criminal prosecution.
Europe/Rest of World Government Regulation
In addition to regulations in the United States, we may be subject to a variety of regulations in other jurisdictions governing, among other things, clinical trials and any commercial sales and distribution of our future product candidates.
Whether or not we obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in foreign countries prior to the commencement of clinical trials or marketing of the product in those countries. Certain countries outside of the United States have a similar process that requires the submission of a clinical trial application much like the IND prior to the commencement of human clinical trials. In Europe, for example, a clinical trial application (“CTA”), must be submitted to each country’s national health authority and an independent ethics committee, much like the FDA and IRB, respectively. Once the CTA is approved in accordance with a country’s requirements, clinical trial development may proceed.
Following the United Kingdom’s exit from the European Union, a separate regulatory regime applies in the United Kingdom to clinical trials and licensing of medicines.
The requirements and process governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. In all cases, the clinical trials are conducted in accordance with cGCPs and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
To obtain regulatory approval of an investigational drug under EU regulatory systems, we must submit a marketing authorization application. The application used to file the NDA in the United States is similar to that required in Europe, with the exception of, among other things, country-specific document requirements.
For other countries outside of the EU, such as countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. In all cases, again, the clinical trials are conducted in accordance with cGCPs and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Authorization Procedures in the European Union
In all cases, the application for marketing approval requires the completion of clinical trials. Clinical trials are currently regulated under Directive 2001/20/EC. EU directives are not directly applicable in the member states. They have to be transposed into national law. National law transposing EU directives often varies to a great extent. However, in April 2014 a new regulation on clinical trials on medicinal products for human use was adopted. Regulations are directly applicable in the member states, so they generally lead to greater harmonization. Regulation 536/2014 (“CTR”), entered into force on in June 2014. The CTR will harmonize the assessment and supervision processes for clinical trials throughout the EU via a Clinical Trials Information System, or CTIS, which will contain a centralized EU portal and database for clinical trials. The exact timing of the Regulation’s application depends on confirmation of full functionality of CTIS through an independent audit. The CTR will become applicable six months after the European Commission publishes notice of this confirmation.
71
Medicines can be authorized in the EU by using either the centralized authorization procedure or national authorization procedures.
| ● | Centralized Procedure (regulated in Regulation (EC) 726/2004). Under the Centralized Procedure a so-called Community Marketing Authorization is issued by the European Commission, based on the opinion of the Committee for Medicinal Products for Human Use of the European Medicines Agency (the “EMA”). The Community Marketing Authorization is valid throughout the entire territory of the European Economic Area (“EEA”) (which includes the 27 Member States of the EU plus Norway, Liechtenstein and Iceland). The Centralized Procedure is mandatory for certain types of products, such as biotechnology medicinal products, orphan medicinal products, and medicinal products indicated for the treatment of AIDS, cancer, neurodegenerative disorders, diabetes, auto-immune and viral diseases. The Centralized Procedure is optional for products containing a new active substance not yet authorized in the EEA, or for products that constitute a significant therapeutic, scientific or technical innovation or which are in the interest of public health in the EU. For medicines that do not fall within these categories, an applicant has the option of submitting an application for a centralized marketing authorization to the EMA, as long as the medicine concerned is a significant therapeutic, scientific or technical innovation, or if its authorization would be in the interest of public health. | |
| ● | Cooperative Authorization Procedures (regulated in Directive 2001/83/EC and implemented into Member States’ national law). There are also two other possible routes to authorize medicinal products in several countries, which are available for investigational drug products that fall outside the scope of the centralized procedure: |
| ○ | Decentralized Procedure. Using the Decentralized Procedure, an applicant may apply for simultaneous authorization in more than one EU country of medicinal products that have not yet been authorized in any EU country and that do not fall within the mandatory scope of the centralized procedure. Under the Decentralized Procedure the applicant chooses one country as Reference Member State. The regulatory authority of the Reference Member State will then be in charge of leading the assessment of the marketing authorization application. |
| ○ | Mutual Recognition Procedure. In the Mutual Recognition Procedure, a medicine is first authorized in one EU Member State, in accordance with the national procedures of that country. Following this, further marketing authorizations can be sought from other EU countries in a procedure whereby the countries concerned agree to recognize the validity of the original, national marketing authorization. |
| ● | Furthermore, there is the option to obtain a national authorization in just one Member State. |
In the EU, upon receiving marketing authorization, new chemical entities generally receive eight years of data exclusivity and an additional two years of market exclusivity. If granted, data exclusivity prevents regulatory authorities in the EU from referencing the innovator’s data to assess a generic application. During the additional two-year period of market exclusivity, a generic marketing authorization can be submitted, and the innovator’s data may be referenced, but no generic product can be marketed until the expiration of the market exclusivity. However, there is no guarantee that a product will be considered by the EU’s regulatory authorities to be a new chemical entity, and there is a risk that products may not qualify for data exclusivity.
UK Regulation
The Medicines and Healthcare products Regulatory Agency (MHRA) is an executive agency of the Department of Health and Social Care in the United Kingdom which is responsible for ensuring that medicines and medical devices work and are acceptably safe.
The MHRA has the following roles:
| ● | Operate post-marketing surveillance – in particular the Yellow Card Scheme – for reporting, investigating and monitoring of adverse drug reactions to medicines and incidents with medical devices. |
| ● | Assess and authorize medicinal products for sale and supply in the UK. |
72
| ● | Oversee the Notified Bodies that ensure medical device manufacturers comply with regulatory requirements before putting devices on the market. |
| ● | Operate a quality surveillance system to sample and test medicines to address quality defects and to monitor the safety and quality of unlicensed products. |
| ● | Investigate internet sales and potential counterfeiting of medicines, and prosecute where necessary. |
| ● | Regulate clinical trials of medicines and medical devices. |
| ● | Monitor and ensure compliance with statutory obligations relating to medicines and medical devices. |
| ● | Promote safe use of medicines and devices. |
The CQC is an executive non-departmental public body of the Department of Health and Social Care of the United Kingdom. It regulates and inspects health and social care services in the UK.
The GPC is the body responsible for the independent regulation of the pharmacy profession within England, Scotland and Wales, responsible for the regulation of pharmacists, pharmacy technicians and pharmacy premises.
Zen Healthcare has established consultants and advisors to ensure it operates in accordance with the Care Quality Commission. Zen Healthcare also has all the regulatory approvals and licenses to operate from the aforementioned bodies and complies with the MHRA, CQC and GPC.
Other Health Care Laws
We may also be subject to healthcare regulation and enforcement by the federal government and the states and foreign governments where we may market our product candidates, if approved. These laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, physician sunshine and privacy and security laws and regulations.
The federal Anti-Kickback Statute prohibits, among other things, any person from knowingly and willfully offering, soliciting, receiving or providing remuneration, directly or indirectly, to induce either the referral of an individual, for an item or service or the purchasing or ordering of a good or service, for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs. The Anti-Kickback Statute is subject to evolving interpretations. In the past, the government has enforced the Anti-Kickback Statute to reach large settlements with healthcare companies based on sham consulting and other financial arrangements with physicians. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act. The majority of states also have anti-kickback laws which establish similar prohibitions and in some cases may apply to items or services reimbursed by any third-party payor, including commercial insurers.
Additionally, the civil False Claims Act prohibits knowingly presenting or causing the presentation of a false, fictitious or fraudulent claim for payment to the United States government. Actions under the False Claims Act may be brought by the Attorney General or as a qui tam action by a private individual in the name of the government. Violations of the False Claims Act can result in very significant monetary penalties and treble damages. The federal government is using the False Claims Act, and the accompanying threat of significant liability, in its investigation and prosecution of pharmaceutical and biotechnology companies throughout the United States, for example, in connection with the promotion of products for unapproved uses and other sales and marketing practices. The government has obtained multi-million and multi-billion dollar settlements under the False Claims Act in addition to individual criminal convictions under applicable criminal statutes. Given the significant size of actual and potential settlements, it is expected that the government will continue to devote substantial resources to investigating healthcare providers’ and manufacturers’ compliance with applicable fraud and abuse laws.
73
HIPAA also created new federal criminal statutes that prohibit among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
There has also been a recent trend of increased federal and state regulation of payments made to physicians and other healthcare providers. The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, (collectively, “the Affordable Care Act”), among other things, imposed new reporting requirements on drug manufacturers for payments made by them to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Failure to submit timely, accurately and completely the required information may result in civil monetary penalties of up to an aggregate of approximately $0.2 million per year (or up to an aggregate of $1.1 million per year for “knowing failures”), for all payments, transfers of value or ownership or investment interests that are not timely, accurately and completely reported in an annual submission. Drug manufacturers are required to submit reports to the government by the 90th day of each calendar year. Certain states also mandate implementation of compliance programs, impose restrictions on drug manufacturer marketing practices and/or require the tracking and reporting of marketing expenditures and pricing information as well as gifts, compensation and other remuneration to physicians.
We may also be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. HIPAA, as amended by HITECH, and their respective implementing regulations, including the final omnibus rule published on January 25, 2013, imposes specified requirements relating to the privacy, security and transmission of individually identifiable health information. Among other things, HITECH makes HIPAA’s privacy and security standards directly applicable to “business associates,” defined as independent contractors or agents of covered entities that create, receive, maintain or transmit protected health information in connection with providing a service for or on behalf of a covered entity. HITECH also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions. In addition, state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways, thus complicating compliance efforts.
Coverage and Reimbursement
Sales of our product candidates, once approved, will depend, in part, on the extent to which the costs of our products will be covered by third-party payors, such as government health programs, private health insurers and managed care organizations. Third-party payors generally decide which drugs they will cover and establish certain reimbursement levels for such drugs. In particular, in the United States, private health insurers and other third-party payors often provide reimbursement for products and services based on the level at which the government (through the Medicare or Medicaid programs) provides reimbursement for such treatments. Patients who are prescribed treatments for their conditions and providers performing the prescribed services generally rely on third-party payors to reimburse all or part of the associated healthcare costs. Patients are unlikely to use our products unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our products. Sales of our products and product candidates, if approved, will therefore depend substantially on the extent to which the costs of products and our product candidates will be paid by third-party payors. Additionally, the market for our products and future product candidates will depend significantly on access to third-party payors’ formularies without prior authorization, step therapy, or other limitations such as approved lists of treatments for which third-party payors provide coverage and reimbursement. Additionally, coverage and reimbursement for therapeutic products can differ significantly from payor to payor. One third-party payor’s decision to cover a particular medical product or service does not ensure that other payors will also provide coverage for the medical product or service, or will provide coverage at an adequate reimbursement rate. As a result, the coverage determination process will require us to provide scientific and clinical support for the use of our products to each payor separately and will be a time-consuming process.
74
In addition, the United States government, state legislatures and foreign governments have continued implementing cost-containment programs, including price controls, restrictions on coverage and reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our future net revenue and results. Decreases in third-party reimbursement for our products and future product candidates or a decision by a third-party payor to not cover our products or future product candidates could reduce physician usage of our products and future product candidates, if approved, and have a material adverse effect on our sales, results of operations and financial condition.
Health Care Reform
In the United States and foreign jurisdictions, there have been a number of legislative and regulatory changes to the healthcare system that could affect our future results of operations. There have been and continue to be a number of initiatives at the United States federal and state levels that seek to reduce healthcare costs.
In particular, in the United States, the Affordable Care Act has had, and is expected to continue to have, a significant impact on the healthcare industry. The Affordable Care Act was designed to expand coverage for the uninsured while at the same time containing overall healthcare costs. The Affordable Care Act, among other things, addressed a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected, increased the minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program and extended the rebate program to individuals enrolled in Medicaid managed care organizations, established annual fees and taxes on manufacturers of certain branded prescription drugs, and established a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts, which, through subsequent legislative amendments, was increased to 70%, off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D. Substantial new provisions affecting compliance were also enacted, which may require us to modify our business practices with healthcare providers and entities.
Since its enactment, there have been judicial and Congressional challenges to certain aspects of the Affordable Care Act. If a law is enacted, many if not all of the provisions of the PPACA may no longer apply to prescription drugs. While we are unable to predict what changes may ultimately be enacted, to the extent that future changes affect how any future products are paid for and reimbursed by government and private payers our business could be adversely impacted. On December 14, 2018, a federal district court in Texas ruled that the PPACA is unconstitutional as a result of the Tax Cuts and Jobs Act, the federal income tax reform legislation previously passed by Congress and signed by President Trump on December 22, 2017, that eliminated the individual mandate portion of the PPACA. The case, Texas, et al, v. United States of America, et al., (N.D. Texas), is an outlier, and the ruling has been stayed by the ruling judge, but in 2019, the Fifth Circuit Court of Appeals subsequently upheld the lower court decision which was then appealed to the United States Supreme Court. The U.S. Supreme Court declined to hear the appeal on an expedited basis and so no decision is expected until the next Supreme Court term in early 2021. We are not able to state with any certainty what will be the impact of this court decision on our business pending further court action and possible appeals. In November 2020, Joseph Biden was elected President and, in January 2021, the Democratic Party obtained control of the Senate. As a result of these electoral developments, it is unlikely that continued legislative efforts will be pursued to repeal PPACA. Instead, it is possible that legislation will be pursued to enhance or reform PPACA. We are not able to state with certainty what the impact of potential legislation will be on our business.
In addition, other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. These changes include aggregate reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013 and, due to subsequent legislative amendments to the statute, will stay in effect through 2025 unless additional Congressional action is taken. Additionally, in January 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, further reduced Medicare payments to several types of providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. Recently there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed bills designed to, among other things, reform government program reimbursement methodologies. Individual states in the United States have also become increasingly active in implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our future product candidates or additional pricing pressures.
75
Facilities and Operational Regulation
U.S.
Federal, state and local regulations (implemented by CMS, FDA, the Occupational Health and Safety Administration (“OSHA”), the DEA, and state departments or boards of public health, public welfare, medicine, nursing, pharmacy, and medical assistance, among others) would require us to meet various standards relating to, among other things, the management, licensing, safety, security and operation of facilities (including, e.g., laboratories, pharmacies, and clinics), personnel qualifications and licensing, the maintenance of proper records, equipment, and quality assurance programs, and the dispensing, storage, and administration of controlled substances. All of our clinics and facilities in the U.S. would be subject to periodic inspection by federal, state and local agencies to determine if the operations, premises, equipment, personnel and patient care meet applicable standards.
Our operations are subject to various federal, state and local hazardous and medical waste disposal laws. As currently in effect, laws governing the disposal of hazardous waste do not classify most of the waste produced in connection with the provision of our health care services as hazardous, although disposal of non-hazardous medical waste is subject to specific state regulation. Our operations are also subject to various air emission and wastewater discharge regulations.
Non-U.S.
We would be subject to a broad spectrum of regulation in other countries. Our operations must comply with various environmental and transportation regulations in the countries in which we operate. Our facilities and clinics are also subject to various standards relating to, among other things, facilities, management, personnel qualifications and licensing, maintenance of proper records, equipment, quality assurance programs, the operation of pharmacies, the protection of workers from blood-borne diseases and the dispensing of controlled substances. All of our operations may be subject to periodic inspection by various governmental authorities to determine if the operations, premises, equipment, personnel and patient care meet applicable standards. Our clinic operations and our related activities generally require licenses, which may be subject to periodic renewal and may be revoked for violation of applicable regulatory requirements.
In addition, many countries impose various investment restrictions on foreign companies. For instance, government approval may be required to enter into a joint venture with a local partner. Some countries do not permit foreign investors to own a majority interest in local companies or require that companies organized under their laws have at least one local stockholder. Investment restrictions therefore affect the corporate structure, operating procedures and other characteristics of our subsidiaries and joint ventures in these and other countries.
Employees
As of December 31, 2020, we had two part time employees and one full time employee, in addition to Zen Healthcare’s staff of over 60 team members across three clinics. None of our employees are represented by a labor union or covered by a collective bargaining agreement. We consider our relationship with our employees to be good.
Facilities
Our principal executive office is located at 1111 Lincoln Road, Suite 500, Miami Beach, FL 33139. We rent approximately 300 square feet of space, which includes our executive offices and research and development operations.
Legal Proceedings
We are not currently subject to any material legal proceedings.
76
Executive Officers, Non-executive employees and Directors
The following table sets forth the name, age as of April 13, 2021, and position of the individuals who serve as directors and executive officers of the Company. The following also includes certain information regarding the individual experience, qualifications, attributes and skills of our directors and executive officers as well as brief statements of those aspects of our directors’ backgrounds that led us to conclude that they are qualified to serve as directors.
| Name | Age | Position | ||
| Executive Officers | ||||
| Dr. Tiago Reis Marques | 44 | Chief Executive Officer and Director | ||
| Stanley M. Gloss | 62 | Chief Financial Officer | ||
| Dr. Yassine Bendiabdallah | 36 | Chief Operating Officer, Director, and Head of UK Clinics | ||
| Non-Employee Directors | ||||
| Prof. Lawrence Steinman | 73 | Executive Chairman and Co-Founder | ||
| Simon Dumesnil | 44 | Director |
Executive Officers
Each executive officer serves at the discretion of our board and holds office until his or her successor is duly elected and qualified or until his or her earlier resignation or removal.
Dr. Tiago Reis Marques (Chief Executive Officer and Director) has served on our board of directors and as Chief Executive Officer since August 2020. He is a senior clinical fellow at Imperial College London and a lecturer at the IoPPN, King’s College London. IoPPN is ranked second in the world for psychology and psychiatry by US News and Best Global Universities, and is home to one of the world’s largest centers for neuroscience research. Dr. Marques is also a psychiatrist at Maudsley Hospital. His research focuses on topics including the mechanism of action of psychiatric medication and novel treatment targets. During his career, he has obtained multiple awards for his research. Dr. Marques is an author or co-author of more than 100 scientific publications in peer-reviewed journals in psychiatry and neuroscience, has co-authored international treatment guidelines and written book chapters, including in the leading book in the field, “Neurobiology of Mental Illness.” We believe that Dr. Marques is qualified to serve on our board of directors due to his medical and scientific background.
Stanley M. Gloss (Chief Financial Officer) has served as our Chief Financial Officer since April 2021. He has been self-employed for the past year doing financial consulting in the areas of accounting and financial reporting. From 2017 to 2020, Mr. Gloss was Controller at Ace Universe, establishing and maintaining the budgets and financial reporting systems and sourcing and maintaining the company insurance. From 2009 to 2016, Mr. Gloss was Controller and Vice President of Finance of Wizard World Inc., where he established and maintained the budgets and financial reporting systems, sourced and maintained the company contracts and insurance, and coordinated public filings. He received his Bachelor of Science in Accounting from Fairfield University.
Dr. Yassine Bendiabdallah (Chief Operating Officer, Head of UK Clinics and Director) has served on our board of directors and as Chief Operating Officer since March 2021. He also co-founded Pasithea Therapeutics Corp. and is currently Head of UK Clinics. Dr. Bendiabdallah is an expert in functional medicine and bio-identical hormone therapy. He completed a Masters in Pharmacy at King’s College London in 2006. He was then awarded a PhD scholarship within Cancer Research UK group at University Colleges London which was completed with honours in 2010. He then went on to work for a number of pharmaceutical companies and held research position at University College London. He has been involved in several startups including HelloDr (HelloDr Ltd, Proximal Health Ltd) an online tech in healthcare, Androgenix Pharmaceuticals Ltd, and Purecare Ltd (Zen Healthcare) which he is the co-founder and current managing director. Zen Healthcare now comprises several clinics and pharmacies in the UK. He also co-founded Pasithea Therapeutics Corp. and is currently Head of UK Clinics. He holds a number of scientific publications in peer-reviewed literature the anticancer research industry. Dr. Bendiabdallah has also attended and presented at several seminars and conferences globally. His current clinical expertise includes age reversal therapies, functional approaches to medicines and intravenous micronutrient therapies. We believe that Dr. Bendiabdallah is qualified to serve on our board of directors due to his significant scientific and industry knowledge.
77
Non-Employee Directors
Prof. Lawrence Steinman has served on our board of directors since August 2020. Prior to joining Pasithea, he served on the Board of Directors of Centocor from 1989 to 1998, the Board of Directors of Neurocine Biosciences from 1997 to 2005, the Board of Directors of Atreca from 2010 to 2019, the Board of Directors of BioAtla from 2016 to the present, and the Board of Directors of Tolerion from 2013 to the present. He is currently the George A. Zimmermann Endowed Chair in the Neurology Department at Stanford University and previously served as the Chair of the Interdepartmental Program in Immunology at Stanford University Medical School from 2003 to 2011. He is a member of the National Academy of Medicine and the National Academy of Sciences. He also founded the Steinman Laboratory at Stanford University, which is dedicated to understanding the pathogenesis of autoimmune diseases, particularly multiple sclerosis and neuromyelitis optica. He received the Frederic Sasse Award from the Free University of Berlin in 1994, the Sen. Jacob Javits Award from the U.S. Congress in 1988 and 2002, the John Dystel Prize in 2004 from the National MS Society in the U.S., the Charcot Prize for Lifetime Achievement in Multiple Sclerosis Research in 2011 from the International Federation of MS Societies and the Anthony Cerami Award in Translational Medicine by the Feinstein Institute of Molecular Medicine in 2015. He also received an honorary Ph.D. at the Hasselt University in 2008. He received his BA (physics) from Dartmouth College in 1968 and his MD from Harvard University in 1973. He also completed a fellowship in chemical immunology at the Weizmann Institute (1974 – 1977) and was an intern and resident at Stanford University Medical School. We believe that Prof. Steinman is qualified to serve on our board of directors due to his extensive background in medicine and his experience as a board member in the life sciences industry.
Simon Dumesnil has served on our board of directors since April 2021. He is currently a Managing Partner and Director of Dunraven Capital Partners Limited, an investment management advisory company incorporated in the UK whose investments are predominately in Eastern European corporate distressed credits and structured products. From 2013 to 2018, Mr. Dumesnil was Managing Director and Head of Structured Financing Group Americas of UBS Securities LLC, where he was responsible for the structured financing trading book in the USA and LATAM and managed a book of financing positions across fixed income products (corporate syndicated and middle-market loans, corporate bonds, real estate loans, CMBS/RMBS/CLO/ABS, LATAM Sovereign). From 2010 to 2013, he was Managing Director and Co-Head Private-Side Structuring Group EMEA of UBS AG., where he was responsible for arranging structured solution transactions and acquisitions for FIG and Special Situation Group (SSG) and also co-headed the illiquid financing business. From 2009 to 2010, Mr. Dumesnil was the Chief Investment Officer Bluestone Capital Management and responsible for investments in distressed assets across Europe. From 2008 to 2009, Mr. Dumesnil was Director of Lehman Brother Holding Inc. and responsible for restructuring and unwinding Lehman Brothers Special Financing Inc. derivative book post-bankruptcy. From 2003 to 2008, Mr. Dumesnil was Director of Lehman Brothers International (Europe). Throughout his career at Dunraven Capital Management, UBS Securities, UBS AG, Bluestone Capital Management and Lehman Brothers, Mr. Dumesnil advised and underwritten corporate risk related to companies across industries or jurisdictions. He has an in-depth knowledge on corporate restructuring and capital structure optimization for companies across their business life cycle. His experience as Chief Investment Officer during the launch and growth phases of a financial services and technology company represents valuable insights for our Company. Mr. Dumesnil attended Cass Business School, where he received his Master of Science in Banking and International Finance and École des Hautes-Études-Commerciales HEC, where he received his Bachelor in Business and Administration, Finance. We believe that Mr. Dumesnil is qualified to serve on our board of directors due to his management and investment experience.
Board Composition and Election of Directors
Our board of directors currently consists of four members. Under our bylaws, the number of directors who shall constitute the Board shall equal not less than 1 nor more than 10, as the Board or majority stockholders may determine by resolution from time to time.
Director Independence
Our board has determined that Dr. Tiago Reis Marques and Dr. Yassine Bendiabdallah currently have relationships that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director, such that neither of them is “independent” as that term is defined under the rules of The Nasdaq Stock Market LLC, or the Nasdaq rules. Our board has determined that Prof. Lawrence Steinman and Simon Dumesnil are both “independent” as that term is defined under the Nasdaq rules. As permitted by Nasdaq, we intend to phase in compliance with Nasdaq’s director independence requirements within the schedule outlined in Nasdaq’s rules. That schedule requires a majority of the members of our Board to be independent within one year of listing. It also requires one member of each Board committee be independent at the time of listing, a majority of Board committee members to be independent within 90 days of listing, and all Board committee members to be independent within one year from listing.
78
Board Elections
In accordance with our bylaws, our stockholders shall elect the directors at our annual meeting of stockholders (except as otherwise provided therein for the filling of vacancies). Each director shall hold office until his death, resignation, retirement, removal, or disqualification, or until his successor shall have been elected and qualified.
Board Leadership Structure
Our board has determined that upon completion of this offering our corporate governance guidelines will provide that, if the chairman of the board is a member of management or does not otherwise qualify as independent, the independent directors of the board may elect a lead director. The lead director’s responsibilities would include, but would not be not limited to: presiding over all meetings of the board of directors at which the chairman is not present, including any executive sessions of the independent directors; approving board meeting schedules and agendas; and acting as the liaison between the independent directors and the chief executive officer and chairman of the board. Our corporate governance guidelines will further provide the flexibility for our board of directors to modify our leadership structure in the future as it deems appropriate.
Role of the Board in Risk Oversight
One of the key functions of our board of directors is informed oversight of our risk management process. Our board of directors will not have a standing risk management committee, but will rather administer this oversight function directly through our board of directors as a whole, as well as through various standing committees of our board of directors that address risks inherent in their respective areas of oversight. In particular, our board of directors is responsible for monitoring and assessing strategic risk exposure and our audit committee has the responsibility to consider and discuss our major financial risk exposures and the steps our management has taken to monitor and control these exposures, including guidelines and policies to govern the process by which risk assessment and management is undertaken. Our audit committee will also monitor compliance with legal and regulatory requirements. Our nominating and corporate governance committee will monitor the effectiveness of our corporate governance practices, including whether they are successful in preventing illegal or improper liability-creating conduct. Our compensation committee will assess and monitor whether any of our compensation policies and programs has the potential to encourage excessive risk-taking. While each committee will be responsible for evaluating certain risks and overseeing the management of such risks, our entire board of directors will be regularly informed through committee reports about such risks.
Board Committees
Following this offering, we will have the following board of directors committees: an audit committee, a compensation committee and a nominating and corporate governance committee. The anticipated composition and responsibilities of each committee are described below. Members will serve on these committees until their resignation or until otherwise determined by our board of directors. Upon our listing on The Nasdaq Capital Market, each committee’s charter will be available under the Corporate Governance section of our website at www.pasithea.com. The reference to our website address does not constitute incorporation by reference of the information contained at or available through our website, and you should not consider it to be a part of this prospectus.
Audit Committee. The audit committee’s responsibilities will include:
| ● | appointing, approving the compensation of, and assessing the independence of our registered public accounting firm; | |
| ● | overseeing the work of our registered public accounting firm, including through the receipt and consideration of reports from such firm; | |
| ● | reviewing and discussing with management and the registered public accounting firm our annual and quarterly financial statements and related disclosures; | |
| ● | coordinating our board of directors’ oversight of our internal control over financial reporting, disclosure controls and procedures and code of business conduct and ethics; |
79
| ● | discussing our risk management policies; | |
| ● | meeting independently with our internal auditing staff, if any, registered public accounting firm and management; | |
| ● | reviewing and approving or ratifying any related person transactions; and | |
| ● | preparing the audit committee report required by SEC rules. |
After this offering, we expect that the initial members of our audit committee will be (chairperson) and . All members of our audit committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC and Nasdaq. Our board has determined that is an audit committee financial expert as defined under the applicable rules of the SEC and has the requisite financial sophistication as defined under the applicable rules and regulations of Nasdaq. Under the rules of the SEC, members of the audit committee must also meet heightened independence standards. However, a minority of the members of the audit committee may be exempt from the heightened audit committee independence standards for one year from the date of effectiveness of the registration statement of which this prospectus forms a part. Our board of directors has determined that is independent under the heightened audit committee independence standards of the SEC and Nasdaq.
As allowed under the applicable rules and regulations of the SEC and Nasdaq, we intend to phase in compliance with the heightened audit committee independence requirements prior to the end of the one-year transition period. The audit committee operates under a written charter that satisfies the applicable standards of the SEC and Nasdaq.
Compensation Committee. The compensation committee’s responsibilities include:
| ● | reviewing and approving, or recommending for approval by the board of directors, the compensation of our Chief Executive Officer and our other executive officers; | |
| ● | overseeing and administering our cash and equity incentive plans; |
| ● | reviewing and making recommendations to our board of directors with respect to director compensation; | |
| ● | reviewing and discussing annually with management our “Compensation Discussion and Analysis,” to the extent required; and | |
| ● | preparing the annual compensation committee report required by SEC rules, to the extent required. |
After this offering, we expect that the members of our compensation committee will be (chair) and . Each of the members of our compensation committee is independent under the applicable rules and regulations of Nasdaq and is a “non-employee director” as defined in Rule 16b-3 promulgated under the Exchange Act. The compensation committee operates under a written charter that satisfies the applicable standards of the SEC and Nasdaq.
Nominating and Corporate Governance Committee. The nominating and corporate governance committee’s responsibilities include:
| ● | identifying individuals qualified to become board members; | |
| ● | recommending to our board of directors the persons to be nominated for election as directors and to each board committee; | |
| ● | developing and recommending to our board of directors corporate governance guidelines, and reviewing and recommending to our board of directors proposed changes to our corporate governance guidelines from time to time; and |
| ● | overseeing a periodic evaluation of our board of directors. |
80
After this offering, we expect that the members of our nominating and corporate governance committee will be (chairperson), and . Each of the members of our nominating and corporate governance committee is an independent director under the applicable rules and regulations of Nasdaq relating to nominating and corporate governance committee independence. The nominating and corporate governance committee operates under a written charter that satisfies the applicable standards of the SEC and Nasdaq.
Compensation Committee Interlocks and Insider Participation
No member of our compensation committee will have been a current or former officer or employee. None of our executive officers served as a director or a member of a compensation committee (or other committee serving an equivalent function) of any other entity, one of whose executive officers served as a director or member of our compensation committee during the last completed fiscal year.
Code of Ethics and Code of Conduct
We have adopted a written code of business conduct and ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. Upon our listing on The Nasdaq Capital Market, our code of business conduct and ethics will be available under the Corporate Governance section of our website at www.pasithea.com. In addition, we intend to post on our website all disclosures that are required by law or the Nasdaq rules concerning any amendments to, or waivers from, any provision of the code. The reference to our website address does not constitute incorporation by reference of the information contained at or available through our website, and you should not consider it to be a part of this prospectus.
81
EXECUTIVE AND DIRECTOR COMPENSATION
Summary Compensation
No compensation was paid to our executive officers, including our Chief Executive Officer (“NEOs”) for services rendered during the year ended December 31, 2020.
Outstanding Equity Awards at Fiscal Year-End
No equity awards were awarded to our NEOs during the year ended December 31, 2020.
Incentive Award Plans
2021 Incentive Plan
Immediately prior to the effectiveness of this offering, we intend to adopt and have approved, the 2021 Incentive Plan. Under the 2021 Incentive Plan, we may grant cash and equity incentive awards to eligible service providers in order to attract, motivate and retain the talent for which we compete. The material terms of the 2021 Incentive Plan, as it is currently contemplated, are summarized below. Until implemented, the terms of the 2021 Incentive Plan and, accordingly, this summary, are subject to change.
Eligibility and Administration
The persons eligible to receive awards under the 2021 Incentive Plan are the officers, directors, employees, consultants and other persons who provide services to the Company on a full-time basis. The foregoing notwithstanding, only full-time employees of the Company, or any parent corporation or subsidiary corporation of the Company (as those terms are defined in Sections 424(e) and (f) of the Code, respectively), shall be eligible for purposes of receiving any incentive stock options (“ISOs”). An employee on leave of absence may be considered as still in the employ of the Company for purposes of eligibility for participation in the 2021 Incentive Plan.
As of December 31, 2020, we had two part time employees and one full time employee eligible to participate in the 2021 Incentive Plan. Because the 2021 Incentive Plan provides for broad discretion in selecting participants and in making awards, the total number of persons who will participate in the 2021 Incentive Plan and the benefits that will be provided to the participants cannot be determined at this time.
82
The 2021 Incentive Plan is to be administered by a committee designated by the Board consisting of not less than two directors, provided, however, that except as otherwise expressly provided in the 2021 Incentive Plan, the Board may exercise any power or authority granted to the compensation committee under the 2021 Incentive Plan. Subject to the terms of the 2021 Incentive Plan, the compensation committee is authorized to select eligible persons to receive awards, determine the type, number and other terms and conditions of, and all other matters relating to, awards, prescribe award agreements (which need not be identical for each participant), and the rules and regulations for the administration of the 2021 Incentive Plan, construe and interpret the 2021 Incentive Plan and award agreements, correct defects, supply omissions or reconcile inconsistencies therein, and make all other decisions and determinations as the compensation committee may deem necessary or advisable for the administration of the 2021 Incentive Plan.
Stock Options and Stock Appreciation Rights
The compensation committee is authorized to grant stock options, including both ISOs, which can result in potentially favorable tax treatment to the participant, and non-qualified stock options, and stock appreciation rights entitling the participant to receive the amount by which the fair market value of a share of Common Stock on the date of exercise exceeds the grant price of the stock appreciation right. The exercise price per share subject to an option and the grant price of a stock appreciation right are determined by the compensation committee, provided that such exercise or grant price shall not be less than 100% of the fair market value of a share on the date of grant. In the case of an ISO, the exercise price per share (to the extent required by the Code at the time of grant) shall not be less than 100% of the fair market value (110% for greater than 10-percent stockholders) of a share on the date of grant. For purposes of the 2021 Incentive Plan, the term “fair market value” means the fair market value of Common Stock, awards or other property as determined by the compensation committee or under procedures established by the compensation committee. Unless otherwise determined by the compensation committee, the fair market value of Common Stock as of any given date shall be the closing sales price per share of Common Stock as reported on the principal stock exchange or market on which Common Stock is traded on the date as of which such value is being determined or, if there is no sale on that date, then on the last previous day on which a sale was reported. The maximum term of each option or stock appreciation right, the times at which each option or stock appreciation right will be exercisable, and provisions requiring forfeiture of unexercised options or stock appreciation rights at or following termination of employment generally are fixed by the compensation committee, except that no option or stock appreciation right may have a term exceeding ten years from the date of grant. An ISO that is granted to a greater than 10-percent stockholder shall have a maximum term of five years. Methods of exercise and settlement and other terms of the options and stock appreciation right are determined by the compensation committee. The compensation committee, thus, may permit the exercise price of options awarded under the 2021 Incentive Plan to be paid in cash, shares, other awards or other property (including loans to participants).
Restricted Stock and Restricted Stock Units
The compensation committee is authorized to grant restricted stock and restricted stock units. Restricted stock is a grant of shares of Common Stock which may not be sold or disposed of, and which shall be subject to such risks of forfeiture and other restrictions as the compensation committee may impose. A participant granted restricted stock generally has all of the rights of a stockholder of the Company, unless otherwise determined by the compensation committee. An award of restricted stock units confers upon a participant the right to receive shares of Common Stock or cash equal to the fair market value of the specified number of shares of Common Stock covered by the restricted stock units at the end of a specified deferral period, subject to such risks of forfeiture and other restrictions as the compensation committee may impose. Prior to settlement, an award of restricted stock units carries no voting or dividend rights or other rights associated with share ownership, although dividend equivalents may be granted, as discussed below.
Dividend Equivalents
The compensation committee is authorized to grant dividend equivalents conferring on participants the right to receive, currently or on a deferred basis, cash, shares of Common Stock, other awards or other property equal in value to dividends paid on a specific number of shares of Common Stock or other periodic payments. Dividend equivalents may be granted alone or in connection with another award, may be paid currently or on a deferred basis and, if deferred, may be deemed to have been reinvested in additional shares of Common Stock, awards or otherwise as specified by the compensation committee.
83
Bonus Stock and Awards in Lieu of Cash Obligations
The compensation committee is authorized to grant shares of Common Stock as a bonus free of restrictions, or to grant shares of Common Stock or other awards in lieu of Company obligations to pay cash under the 2021 Incentive Plan or other plans or compensatory arrangements, subject to such terms as the compensation committee may specify.
Other Stock Based Awards
The compensation committee or the Board is authorized to grant awards that are denominated or payable in, valued by reference to, or otherwise based on or related to shares of Common Stock. The compensation committee determines the terms and conditions of such awards.
Performance Awards
The compensation committee is authorized to grant performance awards to participants on terms and conditions established by the compensation committee. The performance criteria to be achieved during any performance period and the length of the performance period are determined by the compensation committee in its sole discretion upon the grant of the performance award. Performance awards may be valued by reference to a designated number of Shares (in which case they are referred to as performance shares) or by reference to a designated amount of property including cash (in which case they are referred to as performance units). Performance awards may be settled by delivery of cash, shares or other property, or any combination thereof, as determined by the compensation committee. The compensation committee may, in its discretion, determine that the amount payable as a performance award will be reduced from the amount of any potential award.
Section 162(m) Considerations
Section 162(m) of the Internal Revenue Code generally disallows a tax deduction for compensation in excess of $1 million paid in a taxable year by a publicly held corporation to certain executives, including its chief executive officer, chief financial officer, and the next three highly compensated executives of such corporation whose compensation is required to be disclosed in its proxy statement. We expect that, following the offering, our compensation committee will consider the potential effects of Section 162(m) of the Internal Revenue Code on the deductibility of compensation paid to our NEOs, but the compensation committee will have the flexibility to award compensation that is not tax deductible if it determines that such award is in our stockholders’ best interests.
Other Terms of Awards
Awards may be settled in the form of cash, shares of Common Stock, other awards or other property, in the discretion of the compensation committee. The compensation committee may require or permit participants to defer the settlement of all or part of an award in accordance with such terms and conditions as the compensation committee may establish, including payment or crediting of interest or dividend equivalents on deferred amounts, and the crediting of earnings, gains and losses based on deemed investment of deferred amounts in specified investment vehicles. The compensation committee is authorized to place cash, shares of Common Stock or other property in trusts or make other arrangements to provide for payment of the Company’s obligations under the 2021 Incentive Plan. The compensation committee may condition any payment relating to an award on the withholding of taxes and may provide that a portion of any shares of Common Stock or other property to be distributed will be withheld (or previously acquired shares of Common Stock or other property be surrendered by the participant) to satisfy withholding and other tax obligations. Awards granted under the 2021 Incentive Plan generally may not be pledged or otherwise encumbered and are not transferable except by will or by the laws of descent and distribution, or to a designated beneficiary upon the participant’s death, except that the compensation committee may, in its discretion, permit transfers for estate planning or other purposes subject to any applicable restrictions under Rule 16b-3.
Awards under the 2021 Incentive Plan are generally granted without a requirement that the participant pay consideration in the form of cash or property for the grant (as distinguished from the exercise), except to the extent required by law. The compensation committee may, however, grant awards in exchange for other awards under the 2021 Incentive Plan, awards under other Company plans, or other rights to payment from the Company, and may grant awards in addition to and in tandem with such other awards, rights or other awards.
84
Acceleration of Vesting; Change in Control
The compensation committee may, in its discretion, accelerate the exercisability, the lapsing of restrictions or the expiration of deferral or vesting periods of any award, and such accelerated exercisability, lapse, expiration and if so provided in the award agreement or otherwise determined by the compensation committee, vesting shall occur automatically in the case of a “change in control” of the Company, as defined in the 2021 Incentive Plan (including the cash settlement of stock appreciation rights which may be exercisable in the event of a change in control). In addition, the compensation committee may provide in an award agreement that the performance goals relating to any performance award will be deemed to have been met upon the occurrence of any “change in control.”
Amendment and Termination
The Board of Directors may amend, alter, suspend, discontinue or terminate the 2021 Incentive Plan or the compensation committee’s authority to grant awards without further stockholder approval, except that stockholder approval must be obtained for any amendment or alteration if such approval is required by law or regulation or under the rules of any stock exchange or quotation system on which shares of Common Stock are then listed or quoted. Thus, stockholder approval may not necessarily be required for every amendment to the 2021 Incentive Plan which might increase the cost of the 2021 Incentive Plan or alter the eligibility of persons to receive awards.
Stockholder approval will not be deemed to be required under laws or regulations, such as those relating to ISOs, that condition favorable treatment of participants on such approval, although the Board may, in its discretion, seek stockholder approval in any circumstance in which it deems such approval advisable.
The 2021 Incentive Plan will terminate at the earliest of (a) such time as no shares of Common Stock remain available for issuance under the 2021 Incentive Plan,(b) termination of the 2021 Incentive Plan by the Board of Directors, or (c) , 2031.
Securities Laws
The 2021 Incentive Plan is intended to conform to all provisions of the Securities Act, and the Exchange Act and any and all regulations and rules promulgated by the SEC thereunder, including, without limitation, Rule 16b-3. The 2021 Incentive Plan will be administered, and awards will be granted and may be exercised, only in such a manner as to conform to such laws, rules and regulations.
Director Compensation
No compensation was paid to our non-employee directors for services rendered during the year ended December 31, 2020.
The material terms of the non-employee director compensation program, as it is currently contemplated, are summarized below.
The non-employee director compensation program will provide for annual retainer fees and/or long-term equity awards for our non-employee directors. We expect each non-employee director will receive an annual retainer of $ . A non-employee director serving as chairman of the board or lead independent director will receive an additional annual retainer of $ . Non-employee directors serving as the chairs of the audit, compensation and nominating and corporate governance committees will receive additional annual retainers of $ , $ and $ , respectively. Non-employee directors serving as members of the audit, compensation and nominating and corporate governance committees will receive additional annual retainers of $ , $ and $ , respectively. The non-employee directors will also receive initial equity grants of shares of our Common Stock, which shall be subject to vesting requirements, upon initial election to the board of directors. On the date of each annual meeting of our stockholders following the completion of this offering, each non-employee director will receive an annual grant of equity with a pre-determined value, subject to vesting requirements.
In addition, pursuant to the director compensation program, each of our non-employee directors will receive a grant of stock options to purchase shares of our Common Stock pursuant to the 2021 Incentive Plan in connection with this offering, effective as of immediately following the determination of the initial public offering price per share of our Common Stock. These stock options will have an exercise price per share equal to the initial public offering price per share of our Common Stock and will vest on the first anniversary of the date of grant.
Compensation under our non-employee director compensation policy will be subject to the annual limits on non-employee director compensation set forth in the 2021 Incentive Plan, as described above, but such limits will not apply prior to the first calendar year following the calendar year in which this offering is completed. Our board of directors or its authorized committee may modify the non-employee director compensation program from time to time in the exercise of its business judgment, taking into account such factors, circumstances and considerations as it shall deem relevant from time to time, subject to the annual limit on non-employee director compensation set forth in the 2021 Incentive Plan. As provided in the 2021 Incentive Plan, our board of directors or its authorized committee may make exceptions to this limit for individual non-employee directors in extraordinary circumstances, as the board of directors or its authorized committee may determine in its discretion.
85
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following includes a summary of transactions since May 12, 2020 (inception) to which we have been a party in which the amount involved will be the lesser of $120,000 or 1% of our assets, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than 5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest, other than equity and other compensation, termination, change in control and other arrangements, which are described under “Executive and Director Compensation.” We also describe below certain other transactions with our directors, executive officers and stockholders.
Related Party Transactions
Zen Healthcare – Purecare Ltd.
We entered into the Zen Knightsbridge and Holborn Collaboration Agreement with Purecare during the year ended December 31, 2020, whereby both parties have agreed to collaborate on the provision of treatments at Purecare’s London based clinic. The Company has agreed to apply and maintain necessary licenses, market the treatments, and develop and maintain a website for online booking and payments of treatments. Purecare has agreed to provide consulting and treatment rooms at its clinics, as well as providing all pharmaceuticals and equipment necessary for the assessment of patients and provisions of the treatments. All resulting revenue from such treatments shall be allocated 30% to the Company and 70% to Purecare.
Our Chief Operating Officer, Head of UK Clinics and Director, Dr. Yassine Bendiabdallah, is a co-founder, current managing director, and 25% shareholder of Purecare. As of December 31, 2020, no payments have been made pursuant to the Zen Knightsbridge and Holborn Collaboration Agreement.
Zen Healthcare – Portman Health Ltd.
We entered into the Zen Baker Collaboration Agreement with Portman during the year ended December 31, 2020, whereby both parties have agreed to collaborate on the provision of treatments at Portman’s London based clinic. The Company has agreed to apply and maintain necessary licenses, market the treatments, and develop and maintain a website for online booking and payments of treatments. Portman has agreed to provide consulting and treatment rooms at its clinics, as well as providing all pharmaceuticals and equipment necessary for the assessment of patients and provisions of the treatments. All resulting revenue from such treatments shall be allocated 30% to the Company and 70% to Portman.
Dr. Bendiabdallah is a co-founder and 16.25% shareholder of Portman. As of December 31, 2020, no payments have been made pursuant to the Zen Baker Collaboration Agreement.
The IV Doc
On April 9, 2021, Pasithea Clinics Corp. (“Pasithea Clinics”), an affiliate of the Company, entered into a Business Support Services Subcontract (the “Subcontract”) with The IV Doc, pursuant to which The IV Doc will provide certain non-clinical administrative, back office, and other business support services to one or more professional medical practices in the State of New York. During the term of the Subcontract which shall be effective for 15 years from the effective date, Pasithea Clinics will pay The IV Doc monthly subcontract fees in consideration of the subcontract services rendered by The IV Doc. The subcontract fees, which are equal to $22,500 per month, will represent fair market value for the subcontract services and are commensurate with the subcontract services to be provided, and will not constitute an illegal fee-splitting or impermissible profit-sharing arrangement in violation of any applicable laws. In addition to the subcontract fees, Pasithea Clinics will reimburse The IV Doc for all reasonable expenses, including travel, meals and lodging expenses, incurred by The IV Doc in connection with the services provided pursuant to such agreement, provided that such expenses are otherwise commercially reasonable and necessary.
Brio Financial Group
On April 13, 2021, the Company entered into an agreement with Brio Financial Group (“Brio”) pursuant to which Brio will provide Stanley M. Gloss to serve as the Chief Financial Officer of the Company and also provide certain other specified financial and accounting services typically provided by a chief financial officer (the “Brio Agreement”), which are described more fully in the Brio Agreement (the “CFO Services”). The term of the Brio Agreement will run through March 31, 2022, unless terminated by either party upon 10 days prior written notice to the other party, pursuant to the terms of the Brio Agreement. The Company will pay a monthly fixed fee of $7,500 for the CFO Services during the term of the Brio Agreement.
86
Indemnification Agreements
We intend to enter into indemnification agreements with each of our directors and executive officers. These agreements, among other things, require us or will require us to indemnify each director and executive officer to the fullest extent permitted by Delaware law, including indemnification of expenses such as attorneys’ fees, judgments, fines and settlement amounts incurred by the director or executive officer in any action or proceeding, including any action or proceeding by or in right of us, arising out of the person’s services as a director or executive officer. For further information, see “Description of Capital Stock—Limitations on Liability and Indemnification Matters.”
Policies and Procedures for Related Person Transactions
Our board will adopt a written related person transaction policy, to be effective immediately prior to the effectiveness of the registration statement of which this prospectus forms a part, setting forth the policies and procedures for the review and approval or ratification of related person transactions. This policy will cover, with certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act, any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, in which we were or are to be a participant, where the amount involved will be the lesser of $120,000 or 1% of assets the average of our total assets at year-end for the last two completed fiscal years, in any fiscal year and a related person had, has or will have a direct or indirect material interest, including without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person. In reviewing and approving any such transactions, our audit committee is tasked to consider all relevant facts and circumstances, including, but not limited to, whether the transaction is on terms comparable to those that could be obtained in an arm’s length transaction and the extent of the related person’s interest in the transaction. All of the transactions described in this section occurred prior to the adoption of this policy.
87
The following table sets forth information with respect to the beneficial ownership of our Common Stock as of , 2021 by:
| ● | each person, or group of affiliated persons, known by us to beneficially own more than 5% of our outstanding shares of Common Stock (other than NEOs and directors); |
| ● | each of our NEOs; |
| ● | each of our directors; and |
| ● | all of our executive officers and directors as a group. |
The number of shares beneficially owned by each stockholder is determined in accordance with the rules issued by the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under these rules, beneficial ownership includes any shares as to which the individual or entity has sole or shared voting power or investment power, which includes the power to dispose of or to direct the disposition of such security. Except as indicated in the footnotes below, we believe, based on the information furnished to us, that the individuals and entities named in the table below have sole voting and investment power with respect to all shares of Common Stock beneficially owned by them, subject to any community property laws.
Percentage ownership of our Common Stock before this offering is based on 8,307,327 shares of Common Stock outstanding as of April 13, 2021. Percentage ownership of our Common Stock after this offering is based on shares of Common Stock. In computing the number of shares beneficially owned by an individual or entity and the percentage ownership of that person, shares of Common Stock subject to options, restricted units, warrants or other rights held by such person that are currently exercisable or will become exercisable within 60 days of December 31, 2020 are considered outstanding, although these shares are not considered outstanding for purposes of computing the percentage ownership of any other person.
To calculate a stockholder’s percentage of beneficial ownership of Common Stock, we must include in the numerator and denominator those shares of Common Stock, as well as those shares of Common Stock underlying options, warrants and convertible securities, that such stockholder is considered to beneficially own. Shares of Common Stock, and Common Stock underlying options, warrants and convertible securities, held by other stockholders, however, are disregarded in this calculation. Therefore, the denominator used in calculating beneficial ownership of each of the stockholders may be different.
Unless otherwise indicated, the address of each beneficial owner listed below is c/o Pasithea Therapeutics Corp. 1111 Lincoln Road, Suite 500, Miami Beach, FL 33139. To our knowledge, there is no arrangement, including any pledge by any person of securities of the Company, the operation of which may at a subsequent date result in a change in control of the Company.
88
| Beneficial Ownership Before the Offering |
Beneficial Ownership After the Offering |
|||||||||||||
| Common Stock | Common Stock | |||||||||||||
| Name of Beneficial Owner | Shares | % | Shares | % | ||||||||||
| 5% Stockholders: | ||||||||||||||
| Living Trust of Adam Nadelson (1) | 450,000 | 5.4 | % | |||||||||||
| Astatine Capital (2) | 501,250 | 6.0 | % | |||||||||||
| Theseus Capital Ltd. (3) | 501,250 | 6.0 | % | |||||||||||
| EL Capital Inc. (4) | 902,600 | 10.9 | % | |||||||||||
| DPL Capital Inc. (5) | 902,600 | 10.9 | % | |||||||||||
| Craig Auringer | 1,001,000 | 12.1 | % | |||||||||||
| Epic Capital Inc. (6) | 1,051,575 | 12.7 | % | |||||||||||
| Named Executive Officers and Directors: | ||||||||||||||
| Dr. Tiago Reis Marques | 600,000 | 7.2 | % | |||||||||||
| Dr. Yassine Bendiabdallah | 300,000 | 3.6 | % | |||||||||||
| Prof. Lawrence Steinman | 600,000 | 7.2 | % | |||||||||||
| Simon Dumesnil | - | 0 | % | |||||||||||
| Stanley M. Gloss | - | 0 | % | |||||||||||
| All officers and directors as a group (5 persons) | 1,500,000 | 18 | % | |||||||||||
| (1) | Living Trust of Adam Nadelson is a trust for which Adam Nadelson has voting power over. |
| (2) | Astatine Capital is a Cayman Islands company for which Samantha Bauer owns 100% of the membership interests. The address of Astatine Capital is One Capital Place, 3rd Floor, Grand Cayman KY1-1110 Cayman Islands. |
| (3) | Theseus Capital Ltd. is a Cayman Islands company for which Ronald Bauer owns 100% of the membership interests. The address of Theseus Capital Ltd. is One Capital Place, 3rd Floor, Grand Cayman KY1-1110 Cayman Islands. |
| (4) | EL Capital Inc. is a Canadian corporation for which Eric Lazer owns 100% of the membership interests. The address of EL Capital Inc. is 111 Bedford Road, Toronto ON M5R 2K5, Canada. |
| (5) | DPL Capital Inc. is a Canadian corporation for which Dean Lazer owns 100% of the membership interests. The address of DPL Capital Inc. is 169 John Street PH #3 Toronto ON M5T 1X3, Canada. |
| (6) | Epic Capital Inc. is a Nevada Corporation for which Israel Maxx Abramowitz owns 100% of the membership interests. The address of Epic Capital Inc. is 5953 Mabel Road, Unit #138 Las Vegas, NV 89110, United States. |
89
The following description summarizes important terms of our capital stock and certain provisions of our certificate of incorporation and bylaws, each of which will be in effect upon the closing of this offering. Copies of these documents will be filed with the SEC as exhibits to our registration statement, of which this prospectus forms a part.
General
The Company does not have a classified Board of Directors. Pasithea is authorized to issue an aggregate of 500,000,000 shares. The authorized capital stock is divided into 495,000,000 shares of Common Stock having a par value of $0.0001 per share and 5,000,000 shares of preferred stock having a par value of $0.0001 per share. As of April 13, 2021, there were 8,307,327 shares of our Common Stock outstanding held by approximately 46 stockholders of record and no shares of our preferred stock outstanding.
Common Stock
All shares of Common Stock of the Company are one and the same class, identical in all respects and have equal rights, powers and privileges.
Voting. Except as otherwise provided for by resolution of the Board of Directors, the holders of outstanding shares of Common Stock have the exclusive right to vote on all matters requiring stockholder action. On each matter on which holders of Common Stock are entitled to vote, each outstanding share of such Common Stock is entitled to one vote.
Dividends. Subject to the rights of holders of any series of outstanding preferred stock, holders of shares of Common Stock have equal rights of participation in the dividends and other distributions in cash, stock or property of the Company when, as and if declared thereon by the Board of Directors from time to time out of assets or funds of the Company legally available therefor.
Liquidation. Subject to the rights of holders of any series of outstanding preferred stock, holders of shares of Common Stock have equal rights to receive the assets and funds of the Company available for distribution to stockholders in the event of any liquidation, dissolution or winding up of the affairs of the Company, whether voluntary or involuntary.
Rights and Preferences. Holders of our Common Stock will have no preemptive, conversion or subscription rights, and there will be no redemption or sinking funds provisions applicable to our Common Stock. The rights, preferences and privileges of the holders of our Common Stock will be subject to, and may be adversely affected by, the rights of the holders of share of any series of our preferred stock that we may designate and issue in the future.
Fully Paid and Nonassessable. All of our outstanding shares of Common Stock are, and the shares of Common Stock to be issued in this offering will be, fully paid and nonassessable.
Preferred Stock
Shares of preferred stock of the Company may be issued from time to time in one or more series, the shares of each series to have such voting powers, full or limited, if any, and such designations, preferences and relative, participating, optional or other special rights, and qualifications, limitations or restrictions thereof, as are stated and expressed in the resolution or resolutions providing for the issue of such series, adopted by the Board of Directors. The resolutions providing for issuance of any series of preferred stock may provide that such series shall be superior to, rank equally with or be junior to any other series of preferred stock to the extent permitted by law and the terms of any other series of preferred stock.
90
Anti-Takeover Provisions
Some provisions of Delaware law could make the following transactions more difficult: an acquisition of us by means of a tender offer; an acquisition of us by means of a proxy contest or otherwise; or the removal of our incumbent officers and directors. It is possible that these provisions could make it more difficult to accomplish or could deter transactions that stockholders may otherwise consider to be in their best interests or in our best interests, including transactions that provide for payment of a premium over the market price for our shares.
These provisions, summarized below, are intended to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to encourage persons seeking to acquire control of us to first negotiate with our board of directors. We believe that the benefits of the increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages of discouraging these proposals because negotiation of these proposals could result in an improvement of their terms.
Undesignated Preferred Stock. The ability of our board of directors, without action by our stockholders, to issue up to 5,000,000 shares of undesignated preferred stock with voting or other rights or preferences as designated by our board of directors could impede the success of any attempt to effect a change in control of our company. These and other provisions may have the effect of deferring hostile takeovers or delaying changes in control or management of our company.
Requirements for Advance Notification of Stockholder Nominations and Proposals. Our bylaws establish advance notice procedures with respect to stockholder proposals to be brought before a stockholder meeting and the nomination of candidates for election as directors, other than nominations made by or at the direction of our board of directors of a committee of our board of directors.
Limitations on Liability and Indemnification Matters
Our certificate of incorporation limits our directors’ liability to the fullest extent permitted under Delaware law, which prohibits our certificate of incorporation from limiting the liability of our directors for the following:
| ● | any breach of the director’s duty of loyalty to us or our stockholders; |
| ● | acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law; |
| ● | unlawful payment of dividends or unlawful stock repurchases or redemptions; or |
| ● | any transaction from which the director derived an improper personal benefit. |
If Delaware law is amended to authorize corporate action further eliminating or limiting the personal liability of a director, then the liability of our directors will be eliminated or limited to the fullest extent permitted by Delaware law, as so amended.
Our bylaws provide that we will indemnify our directors and officers to the fullest extent permitted under Delaware law and that we shall have the power to indemnify our employees and agents to the fullest extent permitted by law. Our bylaws also permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in this capacity, regardless of whether we would have the power to indemnify such person against such expense, liability or loss under the DGCL.
We also intend to enter into separate indemnification agreements with our directors and executive officers, in addition to indemnification provided for in our bylaws. These agreements, among other things, to provide for indemnification of our directors and executive officers for expenses, judgments, fines and settlement amounts incurred by such persons in any action or proceeding arising out of this person’s services as a director or executive officer or at our request. We believe that these provisions in our certificate of incorporation and bylaws and indemnification agreements are necessary to attract and retain qualified persons as directors and executive officers.
91
The above description of the limitation of liability and indemnification provisions of our certificate of incorporation, our bylaws and our indemnification agreements is not complete and is qualified in its entirety by reference to these documents, each of which will be filed as an exhibit to this registration statement to which this prospectus forms a part.
The limitation of liability and indemnification provisions in our certificate of incorporation and bylaws may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might benefit us and our stockholders. A stockholder’s investment may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
Insofar as indemnification for liabilities under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. There is no pending litigation or proceeding naming any of our directors or officers as to which indemnification is being sought, nor are we aware of any pending or threatened litigation that may result in claims for indemnification by any director or officer.
Listing
We intend to apply to have our Common Stock listed on The Nasdaq Capital Market under the symbol “KTTA”.
Transfer Agent and Registrar
The transfer agent and registrar for our Common Stock will be VStock Transfer, LLC.
92
SHARES ELIGIBLE FOR FUTURE SALE
Immediately prior to this offering, there was no public market for our Common Stock, and no predictions can be made about the effect, if any, that market sales of our Common Stock or the availability of such shares for sale will have on the market price prevailing from time to time. Nevertheless, future sales of our Common Stock in the public market, or the perception that such sales may occur, could adversely affect the market price of our Common Stock and could impair our ability to raise capital through future sales of our securities. See “Risk Factors—Risks Related to this Offering and Ownership of Our Common Stock — Sales of a substantial number of shares of our Common Stock in the public market could cause our stock price to fall.” Furthermore, although we intend to apply to have our Common Stock listed on The Nasdaq Capital Market, we cannot assure you that there will be an active public trading market for our Common Stock.
Upon the closing of this offering, based on the number of shares of our Common Stock outstanding as of , 2021, we will have an aggregate of shares of our Common Stock outstanding. Of these shares of our Common Stock, all of the shares sold in this offering (or shares if the underwriters exercise in full their option to purchase additional shares) will be freely tradable without restriction or further registration under the Securities Act, except for any shares purchased by our “affiliates,” as that term is defined in Rule 144 under the Securities Act, whose sales would be subject to the Rule 144 resale restrictions described below, other than the holding period requirement.
The remaining shares of our Common Stock will be “restricted securities,” as that term is defined in Rule 144 under the Securities Act. These restricted securities are eligible for public sale only if they are registered under the Securities Act or if they qualify for an exemption from registration under Rules 144 or 701 under the Securities Act, which are summarized below. We expect that substantially all of these shares will be subject to the lock-up agreements described below. Upon expiration of the lock-up period, we estimate that approximately shares of our Common Stock will be available for sale in the public market, subject in some cases to applicable volume limitations under Rule 144.
Lock-Up Agreements
All of our directors, executive officers and certain of our security holders are subject to lock-up agreements that, subject to certain exceptions, prohibit them from directly or indirectly offering, pledging, selling, contracting to sell, selling any option or contract to purchase, purchasing any option or contract to purchase, granting any option, right or warrant to purchase or otherwise transferring or disposing of any shares of our Common Stock, options to acquire shares of our Common Stock or any securities convertible into or exercisable or exchangeable for Common Stock, whether now owned or hereafter acquired, or entering into any swap or any other agreement or any transaction that transfer, in whole or in part, directly or indirectly, the economic consequence of ownership, for certain periods of time following the date of this prospectus, without the prior written consent of Kingswood Capital Markets, division of Benchmark Investments, Inc. See the section entitled “Underwriting.”
Rule 144
Affiliate Resales of Restricted Securities. In general, beginning 90 days after the effective date of the registration statement of which this prospectus is a part, a person who is an affiliate of ours, or who was an affiliate at any time during the 90 days before a sale, who has beneficially owned shares of our Common Stock for at least six months would be entitled to sell in “brokers transactions” or certain “riskless principal transactions” or to market makers, a number of shares within any three month-period that does not exceed the greater of:
| ● | 1% of the number of our Common Stock then outstanding, which will equal approximately shares of our Common Stock immediately after this offering; or |
| ● | the average weekly reported trading volume in shares of our Common Stock on The Nasdaq Capital Market during the four calendar weeks preceding the date on which a notice of the sale on Form 144 is filed with the SEC with respect to such sale. |
93
Affiliates resales under Rule 144 are also subject to the availability of current public information about us. In addition, if the number of shares being sold under Rule 144 by an affiliate during any three-month period exceeds 5,000 shares or has an aggregate sale price in excess of $50,000, the seller must file a notice on Form 144 with the SEC and Nasdaq concurrently with either the placing of a sale order with the broker or the execution directly with a market maker.
Non-Affiliate Resales of Restricted Securities. In general, beginning 90 days after the effective date of the registration statement of which this prospectus is a part, a person who is not an affiliate of ours at the time of sale, and has not been an affiliate at any time during the three months preceding a sale, and who has beneficially owned shares of our Common Stock for at least six months but less than a year, is entitled to sell such shares subject only to the availability of current public information about us. If such person has held our shares for at least one year, such person can resell under Rule 144(b)(1) without regard to any Rule 144 restrictions, including the 90-day public company requirement and the current public information requirement.
Non-affiliate resales are not subject to manner of sale, volume limitation or notice filing provisions of Rule 144.
Rule 701
In general, under Rule 701 of the Securities Act, each of our employees, officers, directors, consultants or advisors who purchases shares of our Common Stock from us in connection with a compensatory stock or option plan or other written agreement executed before the effective date of the registration statement under the Securities Act is entitled to resell such shares 90 days after such effective date in reliance on Rule 144. An affiliate of ours can resell shares in reliance on Rule 144 without having to comply with the holding period requirement, and non-affiliates of ours can resell shares in reliance on Rule 144 without having to comply with the current public information and holding period requirements.
The SEC has indicated that Rule 701 will apply to typical options granted by an issuer before it becomes subject to the reporting requirements of the Exchange Act, along with the shares acquired upon exercise of such options, including exercises after an issuer becomes subject to the reporting requirements of the Exchange Act.
Equity Incentive Plans
We intend to file with the SEC a registration statement on Form S-8 under the Securities Act covering the shares of Common Stock reserved for issuance under our 2021 Incentive Plan. The registration statement is expected to be filed and become effective as soon as practicable after the closing of this offering. Accordingly, shares registered under the Form S-8 registration statement will be available for sale in the open market following the registration statement’s effective date, subject to Rule 144 volume limitations and the lock-up agreements described above, if applicable.
94
MATERIAL
U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S. HOLDERS OF OUR
COMMON STOCK
The following discussion is a summary of the material U.S. federal income tax consequences to non-U.S. holders (as defined below) of the purchase, ownership and disposition of our Common Stock issued pursuant to this offering, but does not purport to be a complete analysis of all potential tax effects. The effects of other U.S. federal tax laws, such as estate and gift tax laws, and any applicable state, local or foreign tax laws are not addressed herein. This discussion is based on the Code, Treasury Regulations promulgated thereunder, judicial decisions, and published rulings and administrative pronouncements of the U.S. Internal Revenue Service, or IRS, in effect as of the date of this offering. These authorities may change or be subject to differing interpretations. Any such change or differing interpretation may be applied retroactively in a manner that could adversely affect a non-U.S. holder of our Common Stock. We have not sought and will not seek any rulings from the IRS regarding the matters discussed below. There can be no assurance the IRS or a court will not take a contrary position regarding the tax consequences of the purchase, ownership and disposition of our Common Stock.
This discussion is limited to non-U.S. holders that hold our Common Stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all U.S. federal income tax consequences relevant to a non-U.S. holder’s particular circumstances, including the impact of the alternative minimum tax or the unearned income Medicare contribution tax. In addition, it does not address consequences relevant to holders subject to particular rules, including, without limitation:
| ● | U.S. expatriates and certain former citizens or long-term residents of the United States; |
| ● | persons holding our Common Stock as part of a hedge, straddle or other risk reduction strategy or as part of a conversion transaction or other integrated investment; |
| ● | banks, insurance companies, and other financial institutions; |
| ● | brokers, dealers or traders in securities or currencies; |
| ● | persons that hold more than 5% of our Common Stock, directly or indirectly; |
| ● | “controlled foreign corporations,” “passive foreign investment companies,” and corporations that accumulate earnings to avoid U.S. federal income tax; |
| ● | corporations organized outside of the United States, any state thereof or the District of Columbia that are nonetheless treated as U.S. taxpayers for U.S. federal income tax purposes; |
| ● | partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes (and investors therein); |
| ● | tax-exempt organizations or governmental organizations; |
| ● | persons deemed to sell our Common Stock under the constructive sale provisions of the Code; |
| ● | persons for whom our Common Stock constitutes “qualified small business stock” within the meaning of Section 1202 of the Code; |
| ● | persons who hold or receive our Common Stock pursuant to the exercise of any employee stock option or otherwise as compensation; |
| ● | qualified foreign pension funds as defined in Section 897(l)(2) of the Code and entities all of the interests of which are held by qualified foreign pension funds; |
95
| ● | persons subject to special tax accounting rules as a result of any item of gross income with respect to our Common Stock being taken into account in an applicable financial statement; and |
| ● | tax-qualified retirement plans. |
If a partnership (or other entity treated as a partnership for U.S. federal income tax purposes) holds our Common Stock, the tax treatment of a partner (or person or entity treated as a partner) in the partnership will depend on the status of the partner, the activities of the partnership and certain determinations made at the partner level. Accordingly, partnerships holding our Common Stock and the partners in such partnerships should consult their tax advisors regarding the U.S. federal income tax consequences to them.
THIS DISCUSSION IS FOR INFORMATION PURPOSES ONLY AND IS NOT INTENDED AS LEGAL OR TAX ADVICE. INVESTORS SHOULD CONSULT THEIR TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AS WELL AS ANY TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND DISPOSITION OF OUR COMMON STOCK ARISING UNDER THE U.S. FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE LAWS OF ANY STATE, LOCAL OR NON-U.S. TAXING JURISDICTION OR UNDER ANY APPLICABLE INCOME TAX TREATY.
Definition of a Non-U.S. Holder
For purposes of this discussion, a “non-U.S. holder” is any beneficial owner of our Common Stock that is neither a “U.S. person,” nor an entity treated as a partnership for U.S. federal income tax purposes regardless of its place of organization or formation. A U.S. person is any person that, for U.S. federal income tax purposes, is or is treated as any of the following:
| ● | an individual who is a citizen or resident of the United States; |
| ● | a corporation or other entity created or organized under the laws of the United States, any state thereof, or the District of Columbia and treated as a corporation for U.S. federal income tax purposes; |
| ● | an estate, the income of which is subject to U.S. federal income tax regardless of its source; or |
| ● | a trust that (1) is subject to the primary supervision of a U.S. court and which has one or more U.S. persons (within the meaning of Section 7701(a)(30) of the Code) who have the authority to control all substantial decisions of the trust, or (2) has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person. |
Distributions
As described in the section titled “Dividend Policy,” we do not anticipate declaring or paying dividends to holders of our Common Stock in the foreseeable future. However, if we do make distributions on our Common Stock, such distributions of cash or property on our Common Stock will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Amounts not treated as dividends for U.S. federal income tax purposes will constitute a return of capital and first be applied against and reduce a non-U.S. holder’s adjusted tax basis in its Common Stock, but not below zero. Any excess will be treated as capital gain and will be treated as described below under “—Sale or Other Disposition of Common Stock.”
Subject to the discussion below on effectively connected income, backup withholding and foreign accounts, dividends paid to a non-U.S. holder of our Common Stock that are not effectively connected with the non-U.S. holder’s conduct of a trade or business within the United States will be subject to U.S. federal withholding tax at a rate of 30% of the gross amount of the dividends (or such lower rate specified by an applicable income tax treaty).
96
Non-U.S. holders may be entitled to a reduction in or an exemption from withholding on dividends as a result of either (a) an applicable income tax treaty or (b) the non-U.S. holder holding our Common Stock in connection with the conduct of a trade or business within the United States and dividends being effectively connected with that trade or business. To claim such a reduction in or exemption from withholding, the non-U.S. holder must provide the applicable withholding agent with a properly executed (a) IRS Form W-8BEN or W-8BEN-E (or other applicable documentation) claiming an exemption from or reduction of the withholding tax under the benefit of an income tax treaty between the United States and the country in which the non-U.S. holder resides or is established, or (b) IRS Form W-8ECI stating that the dividends are not subject to withholding tax because they are effectively connected with the conduct by the non-U.S. holder of a trade or business within the United States, as may be applicable. These certifications must be provided to the applicable withholding agent prior to the payment of dividends and must be updated periodically. If a non-U.S. holder holds stock through a financial institution or other agent acting on the holder’s behalf, the holder will be required to provide appropriate documentation to such agent. The holder’s agent will then be required to provide certification to us or our paying agent, either directly or through other intermediaries. Non-U.S. holders that do not timely provide the applicable withholding agent with the required certification, but that qualify for a reduced rate under an applicable income tax treaty, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS.
If dividends paid to a non-U.S. holder are effectively connected with the non-U.S. holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, the non-U.S. holder maintains a permanent establishment in the United States to which such dividends are attributable), then, although exempt from U.S. federal withholding tax (provided the non-U.S. holder provides appropriate certification, as described above), the non-U.S. holder will be subject to U.S. federal income tax on such dividends on a net income basis at the regular U.S. federal income tax rates. In addition, a non-U.S. holder that is a corporation may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on its effectively connected earnings and profits for the taxable year that are attributable to such dividends, as adjusted for certain items. Non-U.S. holders should consult their tax advisors regarding their entitlement to benefits under any applicable income tax treaty.
Sale or Other Disposition of Common Stock
Subject to the discussions below on backup withholding and FATCA, a non-U.S. holder generally will not be subject to U.S. federal income tax on any gain realized upon the sale or other disposition of our Common Stock unless:
| ● | the gain is effectively connected with the non-U.S. holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, the non-U.S. holder maintains a permanent establishment in the United States to which such gain is attributable); |
| ● | the non-U.S. holder is a nonresident alien individual present in the United States for 183 days or more during the taxable year of the disposition and certain other requirements are met; or |
| ● | our Common Stock constitute U.S. real property interests, or USRPIs, by reason of our status as a U.S. real property holding corporation, or USRPHC, for U.S. federal income tax purposes. |
Gain described in the first bullet point above will generally be subject to U.S. federal income tax on a net income basis at the regular U.S. federal income tax rates. A non-U.S. holder that is a foreign corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on its effectively connected earnings and profits, as adjusted for certain items, which will include such effectively connected gain.
A non-U.S. holder described in the second bullet point above will be subject to U.S. federal income tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on any gain derived from the disposition, which may be offset by certain U.S. source capital losses of the non-U.S. holder (even though the individual is not considered a resident of the United States) provided the non-U.S. holder has timely filed U.S. federal income tax returns with respect to such losses.
97
With respect to the third bullet point above, we would be a USRPHC if our USRPIs comprise (by fair market value) at least 50 percent of our business assets. We believe we are not currently and do not anticipate becoming a USRPHC. Because the determination of whether we are a USRPHC depends on the fair market value of our USRPIs relative to the fair market value of our other business assets and our non-U.S. real property interests, however, there can be no assurance we are not a USRPHC or will not become one in the future. Even if we are or were to become a USRPHC, gain arising from the sale or other taxable disposition by a non-U.S. holder of our Common Stock will not be subject to U.S. federal income tax if our Common Stock are “regularly traded,” as defined by applicable Treasury Regulations, on an established securities market, and such non-U.S. holder owned, actually and constructively, 5% or less of our Common Stock throughout the shorter of the five-year period ending on the date of the sale or other taxable disposition or the non-U.S. holder’s holding period. There can be no assurance that our Common Stock will continue to qualify as regularly traded on an established securities market. If any gain on your disposition is taxable because we are a United States real property holding corporation and your ownership of our Common Stock exceeds 5%, you will be taxed on such disposition generally in the manner as gain that is effectively connected with the conduct of a U.S. trade or business (subject to the provisions under an applicable income tax treaty), except that the branch profits tax generally will not apply.
Non-U.S. holders should consult their tax advisors regarding potentially applicable income tax treaties that may provide for different rules.
Information Reporting and Backup Withholding
Subject to the discussion below on FATCA, a non-U.S. holder will not be subject to backup withholding with respect to distributions on our Common Stock we make to the non-U.S. holder, provided the applicable withholding agent does not have actual knowledge or reason to know such holder is a U.S. person and the holder certifies its non-U.S. status, such as by providing a valid IRS Form W-8BEN, W-8BEN-E or W-8ECI, or other applicable certification. However, information returns generally will be filed with the IRS in connection with any distributions (including deemed distributions) made on our Common Stock to the non-U.S. holder, regardless of whether any tax was actually withheld. Such information returns generally include the amount of any such dividends, the name and address of the recipient, and the amount, if any, of tax withheld. A similar report is sent to the holder to whom any such dividends are paid. Copies of these information returns may also be made available under the provisions of a specific treaty or agreement to the tax authorities of the country in which the non-U.S. holder resides or is established.
Information reporting and backup withholding may apply to the proceeds of a sale or other taxable disposition of our Common Stock within the United States, and information reporting may (although backup withholding generally will not) apply to the proceeds of a sale or other taxable disposition of our Common Stock outside the United States conducted through certain U.S.-related financial intermediaries, in each case, unless the beneficial owner certifies under penalty of perjury that it is a non-U.S. holder on IRS Form W-8BEN or W-8BEN-E, or other applicable form (and the payor does not have actual knowledge or reason to know that the beneficial owner is a U.S. person) or such owner otherwise establishes an exemption. Proceeds of a disposition of our Common Stock conducted through a non-U.S. office of a non-U.S. broker generally will not be subject to backup withholding or information reporting.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a non-U.S. holder’s U.S. federal income tax liability, provided the required information is timely furnished to the IRS.
98
Additional Withholding Tax on Payments Made to Foreign Accounts
Withholding taxes may be imposed under Sections 1471 to 1474 of the Code and applicable Treasury Regulations (commonly referred to as the Foreign Account Tax Compliance Act, or FATCA), on certain types of payments made to non-U.S. financial institutions and certain other non-U.S. entities. Specifically, a 30% withholding tax may be imposed on dividends paid on our Common Stock, or (subject to the proposed Treasury Regulations discussed below) gross proceeds from the sale or other disposition of our Common Stock paid to a “foreign financial institution” or a “non-financial foreign entity” (each as defined in the Code) (including, in some cases, when such foreign financial institution or non-financial foreign entity is acting as an intermediary), unless (1) the foreign financial institution undertakes certain diligence and reporting obligations, (2) the non-financial foreign entity either certifies it does not have any “substantial United States owners” (as defined in the Code) or furnishes identifying information regarding each substantial United States owner, or (3) the foreign financial institution or non-financial foreign entity otherwise qualifies for an exemption from these rules. If the payee is a foreign financial institution and is subject to the diligence and reporting requirements in (1) above, it must enter into an agreement with the U.S. Department of the Treasury requiring, among other things, that it undertake to identify accounts held by certain “specified United States persons” or “United States-owned foreign entities” (each as defined in the Code), annually report certain information about such accounts, and withhold 30% on certain payments to non-compliant foreign financial institutions and certain other account holders. Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing FATCA may be subject to different rules.
The withholding provisions under FATCA generally apply to payments of dividends paid on our Common Stock. Further, current provisions of the Code and Treasury Regulations treat gross proceeds from the sale or other disposition of Common Stock as subject to FATCA withholding after December 31, 2018. However, recently proposed Treasury Regulations, if finalized in their present form, would eliminate FATCA withholding on payments of gross proceeds from a sale or other disposition of our Common Stock. In its preamble to such proposed regulations, the U.S. Treasury Department stated that taxpayers generally may rely on these proposed Treasury Regulations until final Treasury Regulations are issued. Prospective investors should consult their tax advisors regarding the potential application of FATCA.
EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN TAX ADVISOR REGARDING THE TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF OUR COMMON STOCK, INCLUDING THE CONSEQUENCES OF ANY RECENT OR PROPOSED CHANGE IN APPLICABLE LAW.
99
Under the terms and subject to the conditions in an underwriting agreement dated the date of this prospectus, the underwriters named below have agreed to purchase, and we have agreed to sell to them, the number of shares indicated below:
| Underwriter | Number of Shares | |||
| Kingswood Capital Markets, division of Benchmark Investments, Inc. | ||||
| Total: | ||||
The underwriters and the representative are collectively referred to as the “underwriters” and the “representative,” respectively. The underwriters are offering the shares of Common Stock subject to their acceptance of the shares from us and subject to prior sale. The underwriting agreement provides that the obligations of the several underwriters to pay for and accept delivery of the shares of Common Stock offered by this prospectus are subject to the approval of certain legal matters by their counsel and to certain other conditions. The underwriters are obligated to take and pay for all of the shares of Common Stock offered by this prospectus if any such shares are taken. However, the underwriters are not required to take or pay for the shares covered by the underwriters’ over-allotment option described below.
The underwriters initially propose to offer part of the shares of Common Stock directly to the public at the offering price listed on the cover page of this prospectus and part to certain dealers at a price that represents a concession not in excess of $ per share under the public offering price. After the initial offering of the shares of Common Stock, the offering price and other selling terms may from time to time be varied by the representative.
Over-Allotment Option
We have granted to the underwriters an option, exercisable for 45 days from the date of this prospectus, to purchase up to additional shares of Common Stock at the public offering price listed on the cover page of this prospectus, less underwriting discounts and commissions. The underwriters may exercise this option solely for the purpose of covering over-allotments, if any, made in connection with the offering of the shares of Common Stock offered by this prospectus. To the extent the option is exercised, each underwriter will become obligated, subject to certain conditions, to purchase about the same percentage of the additional shares of Common Stock as the number listed next to the underwriter’s name in the preceding table bears to the total number of shares of Common Stock listed next to the names of all underwriters in the preceding table.
The following table shows the per share and total public offering price, underwriting discounts and commissions, and proceeds before expenses to us. These amounts are shown assuming both no exercise and full exercise of the underwriters’ option to purchase up to an additional shares of Common Stock.
| Total | ||||||||||||
| Per Share | No Exercise | Full Exercise | ||||||||||
| Public offering price | $ | $ | $ | |||||||||
| Underwriting discounts and commissions to be paid by us | $ | $ | $ | |||||||||
| Proceeds, before expenses, to us | $ | $ | $ | |||||||||
The estimated offering expenses payable by us, exclusive of the underwriting discounts and commissions, are approximately $ . We have agreed to reimburse the underwriters for expenses relating to this offering up to $ .
100
Underwriter Warrants
We have agreed to issue warrants to Kingswood Capital Market, division of Benchmark Investments, Inc., as representative of the underwriters, upon the closing of this offering, which entitle it to purchase up to 5% of the total number of shares of Common Stock being sold in this offering (the “Underwriter Warrants”). The exercise price of the warrants is equal to 100% of the offering price of the Common Stock offered hereby. The Underwriter Warrants will be exercisable at any time and from time to time, in whole or in part, during the four and a half-year period commencing six months from the effective date of this offering. The Underwriter Warrants shall not be redeemable. The Underwriter Warrants and the shares of Common Stock underlying the warrants have been deemed compensation by FINRA and are therefore subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of FINRA. The Underwriter Warrants may not be sold, transferred, assigned, pledged or hypothecated or be the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the securities for a period of 180 days following the effective date of the registration for this offering, except that they may be assigned, in whole or in part, to any officer or partner of the representative, and to members of the underwriting syndicate or selling group (or to officers or partners thereof), or as otherwise permitted, in compliance with FINRA Rule 5110(g)(2). The Underwriter Warrants will contain provisions for one demand registration of the sale of the underlying shares of Common Stock at our expense and unlimited “piggyback” registration rights for a period of five (5) years after the effective date of the registration statement for this offering at our expense. The exercise price and number of shares issuable upon exercise of the Underwriters Warrants may be adjusted in certain circumstances including in the event of a stock split or other corporate events and as otherwise permitted under Rule 5110(f)(2)(G) of FINRA.
The underwriters have informed us that they do not intend sales to discretionary accounts to exceed 5% of the total number of shares of Common Stock offered by them.
We and the underwriters have agreed to indemnify each other against certain liabilities, including liabilities under the Securities Act.
We have also granted Kingswood Capital Markets, division of Benchmark Investments, Inc., a 12-month right of first refusal to act as sole investment banker, sole book-runner, and/or sole placement agent, for any public and private equity and debt offering, including all equity linked financings.
A prospectus in electronic format may be made available on websites maintained by one or more underwriters, or selling group members, if any, participating in this offering. The representative may agree to allocate a number of shares of Common Stock to underwriters for sale to their online brokerage account holders. Internet distributions will be allocated by the representative to underwriters that may make Internet distributions on the same basis as other allocations.
Pricing of the Offering
Prior to this offering, there has been no public market for our Common Stock. The initial public offering price was determined by negotiations between us and the representatives. Among the factors considered in determining the initial public offering price were our future prospects and those of our industry in general, our sales, earnings and certain other financial and operating information in recent periods, and the price-earnings ratios, price-sales ratios, market prices of securities, and certain financial and operating information of companies engaged in activities similar to ours.
At our request, the underwriters have reserved for sale, at the initial public offering price, up to % of the Common Stock in this offering for sale to our directors, officers, employees and other individuals associated with us and members of their families.
Lock-ups
Our directors, executive officers and founders are subject to lock-up agreements that, subject to certain exceptions, prohibit them from directly or indirectly offering, pledging, selling, contracting to sell, selling any option or contract to purchase, purchasing any option or contract to purchase, granting any option, right or warrant to purchase or otherwise transferring or disposing of any shares of our Common Stock, options to acquire shares of our Common Stock or any securities convertible into or exercisable or exchangeable for Common Stock, whether now owned or hereafter acquired, or entering into any swap or any other agreement or any transaction that transfer, in whole or in part, directly or indirectly, the economic consequence of ownership (collectively, the “Prohibited Transactions”), for a period of 365 days following the date of this prospectus, without the prior written consent of Kingswood Capital Markets, division of Benchmark Investments, Inc.; provided, however, if our stock price is above $9.00 per share for 20 of 30 consecutive trading days, then one-third of the securities owned by each of our directors and executive officers will be released from such lock-up restrictions; provided, further however, if our stock price is above $13.50 per share for 20 of 30 consecutive trading days, then two-thirds of the securities owned by each of our directors and executive officers will be released from such lock-up restrictions.
Certain of our security holders are subject to lock-up agreements pursuant to which they may not engage in the Prohibited Transactions for a period of three months following the date of this prospectus without the prior written consent of Kingswood Capital Markets, division of Benchmark Investments, Inc.; provided, however, that an aggregate of 139,064 shares of Common Stock held by certain of our security holders are not subject to the lock-up agreements.
We are also prohibited from engaging in any Prohibited Transactions for a period of 18 months following the date of this prospectus, without the prior written consent of Kingswood Capital Markets, division of Benchmark Investments, Inc.
101
Selling Restrictions
Canada
The shares may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the shares must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 (or, in the case of securities issued or guaranteed by the government of a non-Canadian jurisdiction, section 3A.4) of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Regulation, or each, a Relevant Member State, an offer to the public of any shares of our Common Stock may not be made in that Relevant Member State, except that an offer to the public in that Relevant Member State of any shares of our Common Stock may be made at any time under the following exemptions under the Prospectus Regulation, if they have been implemented in that Relevant Member State:
| (i) | to any legal entity which is a qualified investor as defined in the Prospectus Regulation; |
| (ii) | to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Regulation), subject to obtaining the prior consent of the representatives for any such offer; or |
| (iii) | in any other circumstances falling within Article 1(4) of the Prospectus Regulation, |
provided that no such offer of shares of our Common Stock shall result in a requirement for the publication by us or any underwriter of a prospectus pursuant to Article 3 of the Prospectus Regulation.
For the purposes of this provision, the expression an “offer to the public” in relation to any shares of our Common Stock in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any shares of our Common Stock to be offered so as to enable an investor to decide to purchase any shares of our Common Stock, and the expression “Prospectus Regulation” means Regulation (EU) 2017/1129.
United Kingdom
Each underwriter has represented and agreed that:
| (a) | it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act 2000 (“FSMA) received by it in connection with the issue or sale of the shares of our Common Stock in circumstances in which Section 21(1) of the FSMA does not apply to us; and |
| (b) | it has complied and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the shares of our Common Stock in, from or otherwise involving the United Kingdom. |
102
Hong Kong
Shares of our Common Stock may not be offered or sold by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), (ii) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder or (iii) in other circumstances which do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), and no advertisement, invitation or document relating to shares of our Common Stock may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to shares of our Common Stock which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder.
Japan
No registration pursuant to Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948, as amended) (the “FIEL”) has been made or will be made with respect to the solicitation of the application for the acquisition of the shares of Common Stock.
Accordingly, the shares of Common Stock have not been, directly or indirectly, offered or sold and will not be, directly or indirectly, offered or sold in Japan or to, or for the benefit of, any resident of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan) or to others for re-offering or re-sale, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan except pursuant to an exemption from the registration requirements, and otherwise in compliance with, the FIEL and the other applicable laws and regulations of Japan.
For Qualified Institutional Investors (“QII”)
Please note that the solicitation for newly-issued or secondary securities (each as described in Paragraph 2, Article 4 of the FIEL) in relation to the shares of Common Stock constitutes either a “QII only private placement” or a “QII only secondary distribution” (each as described in Paragraph 1, Article 23-13 of the FIEL). Disclosure regarding any such solicitation, as is otherwise prescribed in Paragraph 1, Article 4 of the FIEL, has not been made in relation to the shares of Common Stock. The shares of Common Stock may only be transferred to QIIs.
For Non-QII Investors
Please note that the solicitation for newly-issued or secondary securities (each as described in Paragraph 2, Article 4 of the FIEL) in relation to the shares of Common Stock constitutes either a “small number private placement” or a “small number private secondary distribution” (each as is described in Paragraph 4, Article 23-13 of the FIEL). Disclosure regarding any such solicitation, as is otherwise prescribed in Paragraph 1, Article 4 of the FIEL, has not been made in relation to the shares of Common Stock. The shares of Common Stock may only be transferred en bloc without subdivision to a single investor.
Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of shares of our Common Stock may not be circulated or distributed, nor may the shares of our Common Stock be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore, or the SFA, (ii) to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where shares of our Common Stock are subscribed or purchased under Section 275 by a relevant person which is: (i) a corporation (which is not an accredited investor) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or (ii) a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary is an accredited investor, shares, debentures and units of shares and debentures of that corporation or the beneficiaries’ rights and interest in that trust shall not be transferable for 6 months after that corporation or that trust has acquired shares of our Common Stock under Section 275 except: (a) to an institutional investor under Section 274 of the SFA or to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA; (b) where no consideration is given for the transfer; or (c) by operation of law.
103
The validity of the shares of Common Stock offered hereby and certain other legal matters will be passed upon for us by McDermott Will & Emery LLP. Certain legal matters in connection with this offering will be passed upon for the underwriters by Sheppard, Mullin, Richter & Hampton LLP.
Marcum LLP, our independent registered public accounting firm, has audited our financial statements at December 31, 2020 and for the period from May 12, 2020 (inception) to December 31, 2020, as set forth in their report. We have included our financial statements in the prospectus and elsewhere in the registration statement in reliance on Marcum LLP’s report, given on their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the Securities and Exchange Commission a registration statement on Form S-1 under the Securities Act with respect to the shares of Common Stock offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules filed therewith. For further information about us and the shares of Common Stock offered hereby, we refer you to the registration statement and the exhibits and schedules filed thereto. Statements contained in this prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the registration statement. Upon completion of this offering, we will be required to file periodic reports, proxy statements, and other information with the Securities and Exchange Commission pursuant to the Exchange Act. You may obtain information on the operation of the public reference rooms by calling the Securities and Exchange Commission at 1-800-SEC-0330. The Securities and Exchange Commission also maintains an Internet website that contains reports, proxy statements and other information about registrants, like us, that file electronically with the Securities and Exchange Commission. The address of that site is www.sec.gov.
104
INDEX TO FINANCIAL STATEMENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors of Pasithea Therapeutics Corp.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Pasithea Therapeutics Corp. (the “Company”) as of December 31, 2020, and the related consolidated statements of operations, changes in stockholders’ equity and cash flows for the period from May 12, 2020 (inception) through December 31, 2020, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020, and the results of its operations and its cash flows for the period from May 12, 2020 (inception) through December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph – Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 3 to the financial statements, the Company’s ability to execute its business plan is dependent upon its completion of the proposed initial public offering described in the financial statements, other issuances of equity securities, obtaining debt financing, or increasing sales. The Company lacks the financial resources it needs to sustain operations for a reasonable period of time, which is considered to be one year from the issuance date of the financial statements. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 3. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ Marcum LLP
Marcum LLP
We have served as the Company’s auditor since 2021.
New Haven, CT
April 13, 2021
F-2
CONSOLIDATED BALANCE SHEET
| December 31, 2020 | ||||
| ASSETS | ||||
| Current assets: | ||||
| Cash and cash equivalents | $ | 243,650 | ||
| Prepaid expenses | 4,308 | |||
| Total current assets | 247,958 | |||
| Total assets | $ | 247,958 | ||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||
| Current liabilities: | ||||
| Accounts payable and accrued liabilities | $ | 6,603 | ||
| Total current liabilities | 6,603 | |||
| Total liabilities | 6,603 | |||
| Commitments and Contingencies (Note 4) | ||||
| Stockholders’ equity: | ||||
| Preferred stock, par value $0.0001, 5,000,000 shares authorized; 0 issued and outstanding | - | |||
| Common stock, par value $0.0001, 495,000,000 shares authorized; 7,469,125 shares issued and outstanding as of December 31, 2020 | 14,938 | |||
| Additional paid-in capital | 267,401 | |||
| Accumulated deficit | (40,984 | ) | ||
| Total stockholders’ equity | 241,355 | |||
| Total liabilities and stockholders’ equity | $ | 247,958 | ||
The accompanying notes are an integral part of the consolidated financial statements.
F-3
CONSOLIDATED STATEMENT OF OPERATIONS
| For the Period from Inception (May 12, 2020) Through December 31, 2020 | ||||
| Operating expenses: | ||||
| Selling, general and administrative | $ | 40,984 | ||
| Loss from operations | (40,984 | ) | ||
| Loss before income taxes | (40,984 | ) | ||
| Benefit from (provision for) income taxes | - | |||
| Net income (loss) | $ | (40,984 | ) | |
| Weighted-average common shares outstanding, basic and diluted | 7,364,166 | |||
| Basic and diluted net loss per common share | $ | (0.00 | ) | |
The accompanying notes are an integral part of the consolidated financial statements.
F-4
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY
| Additional | Total Stockholder’s | |||||||||||||||||||
| Common Stock | Paid-in | Accumulated | Equity | |||||||||||||||||
| Shares | Amount | Capital | Deficit | (Deficit) | ||||||||||||||||
| Balance, May 12, 2020 (inception) | - | $ | - | - | - | $ | - | |||||||||||||
| Issuance of common stock for cash | 7,300,000 | 14,600 | - | - | 14,600 | |||||||||||||||
| Issuance of common stock for cash | 156,250 | 313 | 246,826 | - | 247,139 | |||||||||||||||
| Issuance of common stock for cash | 12,875 | 25 | 20,575 | - | 20,600 | |||||||||||||||
| Net loss | - | - | - | (40,984 | ) | (40,984 | ) | |||||||||||||
| Balance at December 31, 2020 | 7,469,125 | $ | 14,938 | $ | 267,401 | $ | (40,984 | ) | $ | 241,355 | ||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-5
CONSOLIDATED STATEMENT OF CASH FLOWS
| For the Period from Inception (May 12, 2020) Through December 31, 2020 | ||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||
| Net loss | $ | (40,984 | ) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||
| Changes in operating assets and liabilities: | ||||
| Changes in prepaid expenses | (4,308 | ) | ||
| Changes in accounts payable and accrued liabilities | 6,603 | |||
| Net cash used in operating activities | (38,689 | ) | ||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||
| Cash proceeds from issuance of common stock | 282,339 | |||
| 282,339 | ||||
| NET CHANGE IN CASH | 243,650 | |||
| Cash - Beginning of period | - | |||
| Cash - End of period | $ | 243,650 | ||
The accompanying notes are an integral part of the consolidated financial statements.
F-6
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE PERIOD MAY 12, 2020 (INCEPTION) TO DECEMBER 31, 2020
NOTE 1 – NATURE OF THE ORGANIZATION AND BUSINESS
Pasithea Therapeutics Corp. (“Pasithea” or the “Company”) was incorporated in the State of Delaware on May 12, 2020. The Company is engaged in psychiatric and neurological research regarding CNS disorders with the goal of translating this research into clinic-ready drugs.
The Company’s secondary operations focus on establishing anti-depression clinics across the UK and providing business support services to similar entities in the US, using trained pharmacists to administer intravenous infusions of ketamine. Pasithea has partnered with two successful clinics for immediate exposure in locations across Los Angeles, New York City and London.
The Company is located in Miami Beach, Florida USA.
As of December 31, 2020, the Company had not commenced core operations. All activity for the period from May 12, 2020 (inception) through December 31, 2020 relates to the Company’s formation and raising funds through issuing shares of the Company’s common stock. The Company has selected December 31 as its fiscal year end.
Throughout this report, the terms “our,” “we,” “us,” and the “Company” refer to Pasithea Therapeutics Corp. and its subsidiaries, Pasithea Therapeutics Limited (UK) and Pasithea Clinics Inc. Pasithea Therapeutics Limited (UK) is a private limited Company, registered in the United Kingdom (UK). Pasithea Clinics Inc. is incorporated in Delaware.
Basis of Presentation
The accompanying audited consolidated financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
Emerging Growth Company
The Company is an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and it may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s unaudited condensed financial statements with another public company which is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Basis of Consolidation
The consolidated financial statements include the consolidated financial statements of the Company and our wholly owned subsidiaries, Pasithea Therapeutics Limited (UK) and Pasithea Clinics Inc. All inter-company balances and transactions among the companies have been eliminated upon consolidation.
F-7
COVID-19 Pandemic
In March 2020, the World Health Organization characterized the outbreak of the novel strain of coronavirus, specifically identified as COVID-19, as a global pandemic. This has resulted in governments enacting emergency measures to combat the spread of the virus. These measures, which include the implementation of travel bans, self-imposed quarantine periods and social distancing, have caused material disruption to business, resulting in a global economic slowdown. Equity markets have experienced significant volatility and weakness and the governments and central banks have reacted with significant monetary and fiscal interventions designed to stabilize economic conditions.
The current challenging economic climate may lead to adverse changes in cash flows, working capital levels and/or debt balances, which may also have a direct impact on the Company’s operating results and financial position in the future. The ultimate duration and magnitude of the impact and the efficacy of government interventions on the economy and the financial effect on the Company is not known at this time. The extent of such impact will depend on future developments, which are highly uncertain and not in the Company’s control, including new information which may emerge concerning the spread and severity of COVID-19 and actions taken to address its impact, among others. The repercussions of this health crisis could have a material adverse effect on the Company’s business, financial condition, liquidity and operating results.
In response to COVID-19, the Company has implemented working practices to address potential impacts to its operations, employees and customers, and will take further measures in the future if and as required. At present, we do not believe there has been any appreciable impact on the Company specifically associated with COVID-19.
NOTE 2 – ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation
The Company evaluates the need to consolidate affiliates based on standards set forth in ASC 810 Consolidation (“ASC 810”).
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries, Pasithea Therapeutics Limited (UK) and Pasithea Clinics Inc. All significant consolidated transactions and balances have been eliminated in consolidation.
These consolidated financial statements are presented in U.S. Dollars.
Significant Accounting Policies
Use of Estimates
The preparation of financial statement in conformity with GAAP requires the Company’s management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statement and the reported amounts of revenues and expenses during the reporting period.
Making estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the financial statement, which management considered in formulating its estimate, could change in the near term due to one or more future confirming events. Accordingly, the actual results could differ significantly from those estimates.
Cash and cash equivalents
The Company considers all short-term investments with an original maturity of three months or less when purchased to be cash equivalents. As of December 31, 2020, we had no cash balances in bank deposit accounts that exceeded federally insured limits.
F-8
Income Taxes
The Company follows the asset and liability method of accounting for income taxes under ASC 740, “Income Taxes.” Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that included the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
ASC 740 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities. The Company recognizes accrued interest and penalties related to unrecognized tax benefits as income tax expense. There were no unrecognized tax benefits and no amounts accrued for interest and penalties as of December 31, 2020. The Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation from its position. The Company is subject to income tax examinations by major taxing authorities since inception.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist of a cash account in a financial institution, which, at times, may exceed the Federal Depository Insurance Coverage of $250,000. As of December 31, 2020, the Company has not experienced losses on this account and management believes the Company is not exposed to significant risks on such account.
Fair Value of Financial Instruments
The fair value of the Company’s assets and liabilities, which qualify as financial instruments under ASC 820, “Fair Value Measurements and Disclosures,” approximates the carrying amounts represented in the accompanying balance sheet, primarily due to their short-term nature.
Net Loss Per Share
Net loss per share is computed by dividing net loss by the weighted average number of common shares outstanding during the reporting period. Diluted earnings per share is computed similar to basic earnings per share, except the weighted average number of common shares outstanding are increased to include additional shares from the assumed exercise of share options, if dilutive. There are no outstanding dilutive or potentially dilutive instruments.
Recent Accounting Pronouncements
Management does not believe that any recently issued, but not yet effective, accounting pronouncements, if currently adopted, would have a material effect on the Company’s financial statement.
F-9
NOTE 3 – GOING CONCERN AND MANAGEMENT’S LIQUIDITY PLANS
As of December 31, 2020, the Company had $243,650 in its operating bank account, and working capital of $241,355. The Company’s liquidity needs up to December 31, 2020 had been satisfied through proceeds from the issuance of common stock.
The accompanying consolidated financial statements have been prepared on the basis that the Company will continue as a going concern, which assumes the realization of assets and the satisfaction of liabilities in the normal course of business. As of December 31, 2020, the Company has an accumulated deficit of $40,984 and has experienced losses from continuing operations. Based on the Company’s cash balance as of December 31, 2020, and projected cash needs for 2021, management estimates that it will need to increase sales revenue and/or raise additional capital to cover operating and capital requirements. Management will need to raise the additional funds through issuing additional shares of common stock or other equity securities or obtaining debt financing. Although management has been successful to date in raising necessary funding, there can be no assurance that sales revenue will substantially increase or that any required future financing can be successfully completed on a timely basis, or on terms acceptable to the Company. Based on these circumstances, management has determined that these conditions raise substantial doubt about the Company’s ability to continue as a going concern.
Accordingly, the accompanying consolidated financial statements have been prepared in conformity with U.S. GAAP, which contemplates continuation of the Company as a going concern and the realization of assets and the satisfaction of liabilities in the normal course of business. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
NOTE 4 – COMMITMENTS AND CONTINGENCIES
Collaboration Agreement – Zen Baker Street Clinic (UK)
During the year ended December 31, 2020, the Company entered into a Collaboration Agreement with Portman Health Ltd, (UK) (“Portman”), whereby both parties have agreed to collaborate on the provision of treatments at Portman’s London based clinic. The Company has agreed to apply and maintain necessary licenses, market the treatments, and develop and maintain a website for online booking and payments of treatments. Portman Health has agreed to provide consulting and treatment rooms at its clinics, as well as providing all pharmaceuticals and equipment necessary for the assessment of patients and provisions of the treatments. All resulting revenue from such treatments shall be allocated 30% to the Company and 70% to Portman.
Collaboration Agreement – Zen Knightsbridge Clinic (UK)
During the year ended December 31, 2020, the Company entered into a Collaboration Agreement with Purecare Limited (UK) (“Purecare”), whereby both parties have agreed to collaborate on the provision of treatments at Purecare’s London based clinic. The Company has agreed to apply and maintain necessary licenses, market the treatments, and develop and maintain a website for online booking and payments of treatments. Purecare has agreed to provide consulting and treatment rooms at its clinics, as well as providing all pharmaceuticals and equipment necessary for the assessment of patients and provisions of the treatments. All resulting revenue from such treatments shall be allocated 30% to the Company and 70% to Purecare.
Business Support Services Subcontract – The IV Doc
On April 9, 2021, Pasithea Clinics Corp. (“Pasithea Clinics”), an affiliate of the Company, entered into a Business Support Services Subcontract (the “Subcontract”) with The IV Doc, pursuant to which The IV Doc will provide certain non-clinical administrative, back office, and other business support services to one or more professional medical practices in the State of New York. During the term of the Subcontract which shall be effective for 15 years from the effective date, Pasithea Clinics will pay The IV Doc monthly subcontract fees in consideration of the subcontract services rendered by The IV Doc. The subcontract fees, which are equal to $22,500 per month, will represent fair market value for the subcontract services and are commensurate with the subcontract services to be provided, and will not constitute an illegal fee-splitting or impermissible profit-sharing arrangement in violation of any applicable laws. In addition to the subcontract fees, Pasithea Clinics will reimburse The IV Doc for all reasonable expenses, including travel, meals and lodging expenses, incurred by The IV Doc in connection with the provision of the subcontract services, provided that such expenses are otherwise commercially reasonable and necessary.
F-10
NOTE 5 – STOCKHOLDERS’ EQUITY
The Company is authorized to issue an aggregate of 500,000,000 shares. The authorized capital stock is divided into: (i) 495,000,000 shares of common stock having a par value of $0.0001 per share and (ii) 5,000,000 shares of preferred stock having a par value of $0.0001 per share.
From inception, May 12, 2020 through December 31, 2020, the Company issued 7,300,000 shares of common stock at a price of $0.0001 per share and 156,250 shares of common stock at a price of $0.08 per share for total cash of approximately of $261,739, which is net of share issuance costs of $2,861.
During the period, several investors advanced funds totaling approximately $20,600 to the Company with no specific terms of repayment, interest or maturity, subsequent to which the parties executed conversion documents to convert the funds into common shares. As the fair value of the equity instruments was equal to the funds advanced, there was no gain or loss on the transaction when on December 30, 2020, the Company issued 12,875 shares of common stock at a price of $0.08 per share to the respective investors.
NOTE 6 – INCOME TAXES
The Company accounts for income taxes under ASC 740 - Income Taxes (“ASC 740”), which provides for an asset and liability approach of accounting for income taxes. Under this approach, deferred tax assets and liabilities are recognized based on anticipated future tax consequences, using currently enacted tax laws, attributed to temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts calculated for income tax purposes.
Significant components of the Company’s deferred tax assets as of December 31, 2020 are summarized below.
| December 31, 2020 | ||||
| Deferred tax assets: | ||||
| Net operation loss carryforwards | $ | 11,000 | ||
| Total deferred tax asset | 11,000 | |||
| Valuation allowance | (11,000 | ) | ||
| $ | - | |||
The Company recognizes deferred tax assets to the extent that it believes that these assets are more likely than not to be realized. In making such a determination, the Company considers all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, tax-planning strategies, and results of recent operations. The Company assessed the need for a valuation allowance against its net deferred tax assets and determined a full valuation allowance is required because it is more likely than not that all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Based upon the level of historical taxable income and projections for future taxable income over the periods in which the DTAs are deductible, management believes it is more likely than not that the Company will not realize the benefits of these deductible differences as of December 31, 2020. The Company has no history of generating taxable income. Therefore, a valuation allowance of $11,000 was recorded as of December 31, 2020. Deferred tax assets were calculated using the Company’s combined effective tax rate, which it estimated to be 28%. The effective rate is reduced to 0% for 2020 due to the full valuation allowance on its net deferred tax assets.
The Company’s ability to utilize net operating loss carryforwards will depend on its ability to generate adequate future taxable income. Future utilization of the net operating loss carry forwards is subject to certain limitations under Section 382 of the Internal Revenue Code. As of December 31, 2020, the Company had net operating loss carryforwards available to offset future taxable income in the amounts of approximately $41,000.
The Company has evaluated its income tax positions and has determined that it does not have any uncertain tax positions. The Company will recognize interest and penalties related to any uncertain tax positions through its income tax expense.
The Company is subject to franchise tax filing requirements in the State of Delaware.
F-11
NOTE 7 – SUBSEQUENT EVENTS
Subsequent to December 31, 2020, the Company entered into various subscription agreements in connection with its private placement (the “Offering”) seeking to raise up to $1 million through the sale of 625,000 shares of the Company’s common stock, at a price of $0.08 per share, with a closing date for accepted subscriptions of January 31, 2021. In 2021 to date, the Company issued a total of 395,625 shares for aggregate proceeds received of approximately $633,000 related to the Offering.
In 2021 to date, the Company entered into various subscription agreements in connection with its private placement (the “Offering 2”) seeking to raise up to $5 million through the sale of 2,083,333 shares of the Company’s common stock, at a price of $0.12 per share, with a closing date for accepted subscriptions of March 31, 2021. The Company reserves the right to extend the closing date at the board of directors’ discretion. In 2021 to date, the Company issued a total of 239,969 shares for aggregate proceeds received of approximately $576,000 related to the Offering 2.
Effective April 8, 2021, we amended our certificate of incorporation to effect a 1-for-20 reverse stock split of our outstanding shares of Common Stock. No fractional shares will be issued as a result of the reverse stock split. Any fractional shares resulting from the reverse stock split shall be paid in cash. The reverse stock split does not otherwise affect any of the rights currently accruing to holders of our Common Stock. All share information presented in this prospectus (including the financial statements) has been retroactively adjusted to reflect the reduced number of shares outstanding.
The Company has evaluated subsequent events through the date the consolidated financial statements are available to be issued. Other than the matters described above, there are no subsequent events identified that would require disclosure in the consolidated financial statements.
F-12

Pasithea Therapeutics Corp.
SHARES OF COMMON STOCK
PROSPECTUS
KINGSWOOD CAPITAL MARKETS
division of Benchmark Investments, Inc.
, 2021
Through and including , 2021 (the 25th day after the date of this offering), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
Part II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table indicates the expenses to be incurred in connection with the offering described in this registration statement, other than underwriting discounts and commissions, all of which will be paid by us. All amounts are estimated except the Securities and Exchange Commission registration fee, the Financial Industry Regulatory Authority, Inc., or FINRA, filing fee and the Nasdaq listing fee.
| Amount | ||||
| Securities and Exchange Commission registration fee | $ | 2,182 | ||
| FINRA filing fee | * | |||
| Initial listing fee | * | |||
| Accountants’ fees and expenses | * | |||
| Legal fees and expenses | * | |||
| Blue Sky fees and expenses | * | |||
| Transfer Agent’s fees and expenses | * | |||
| Printing and engraving expenses | * | |||
| Miscellaneous | * | |||
| Total expenses | $ | * | ||
| * | To be filed by amendment. |
Item 14. Indemnification of Directors and Officers.
Section 102 of the DGCL permits a corporation to eliminate the personal liability of directors of a corporation to the corporation or its stockholders for monetary damages for a breach of fiduciary duty as a director, except where the director breached his duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law or obtained an improper personal benefit. Our certificate of incorporation provides that no director of the Registrant shall be personally liable to it or its stockholders for monetary damages for any breach of fiduciary duty as a director, notwithstanding any provision of law imposing such liability, except to the extent that the DGCL prohibits the elimination or limitation of liability of directors for breaches of fiduciary duty.
Section 145 of the DGCL provides that a corporation has the power to indemnify a director, officer, employee, or agent of the corporation, or a person serving at the request of the corporation for another corporation, partnership, joint venture, trust or other enterprise in related capacities against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with an action, suit or proceeding to which he was or is a party or is threatened to be made a party to any threatened, ending or completed action, suit or proceeding by reason of such position, if such person acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation, and, in any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful, except that, in the case of actions brought by or in the right of the corporation, no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or such other court shall deem proper.
II-1
Our certificate of incorporation provides that we will indemnify each person who was or is a party or threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than an action by or in the right of us) by reason of the fact that he or she is or was, or has agreed to become, a director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise (all such persons being referred to as an “Indemnitee”), or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding and any appeal therefrom, if such Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, and, with respect to any criminal action or proceeding, he or she had no reasonable cause to believe his or her conduct was unlawful. Our certificate of incorporation provides that we will indemnify any Indemnitee who was or is a party to an action or suit by or in the right of us to procure a judgment in our favor by reason of the fact that the Indemnitee is or was, or has agreed to become, a director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise, or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees) and, to the extent permitted by law, amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding, and any appeal therefrom, if the Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, except that no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to us, unless a court determines that, despite such adjudication but in view of all of the circumstances, he or she is entitled to indemnification of such expenses. Notwithstanding the foregoing, to the extent that any Indemnitee has been successful, on the merits or otherwise, he or she will be indemnified by us against all expenses (including attorneys’ fees) actually and reasonably incurred in connection therewith. Expenses must be advanced to an Indemnitee under certain circumstances.
We intend to enter into indemnification agreements with each of our directors and officers. These indemnification agreements may require us, among other things, to indemnify our directors and officers for some expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by a director or officer in any action or proceeding arising out of his or her service as one of our directors or officers, or any other company or enterprise to which the person provides services at our request.
We maintain a general liability insurance policy that covers certain liabilities of directors and officers of our corporation arising out of claims based on acts or omissions in their capacities as directors or officers.
In any underwriting agreement we enter into in connection with the sale of Common Stock being registered hereby, the underwriters will agree to indemnify, under certain conditions, us, our directors, our officers and persons who control us within the meaning of the Securities Act, against certain liabilities.
Item 15. Recent Sales of Unregistered Securities.
Set forth below is information regarding unregistered securities issued by us within the past three years. Also included is the consideration received by us for such unregistered securities and information relating to the section of the Securities Act, or rule of the Securities and Exchange Commission, under which exemption from registration was claimed.
| 1. | Subsequent to December 31, 2020, the Company entered into various subscription agreements in connection with its private placement (the “Offering”) seeking to raise up to $1 million through the sale of 625,000 shares of the Company’s Common Stock, at a price of $0.08 per share, with a closing date for accepted subscriptions of January 31, 2021. In 2021 to date, the Company issued a total of 395,625 shares for aggregate proceeds received of approximately $633,000 related to the Offering. |
| 2. | In 2021 to date, the Company entered into various subscription agreements in connection with its private placement (the “Offering 2”) seeking to raise up to $5 million through the sale of 2,083,333 shares of the Company’s Common Stock, at a price of $0.12 per share, with a closing date for accepted subscriptions of March 31, 2021. The Company reserves the right to extend the closing date at the board of directors’ discretion. In 2021 to date, the Company issued a total of 239,969 shares for aggregate proceeds received of approximately $1,200,000 related to the Offering 2. |
II-2
The offer and sale of all securities listed in this item 15 was made to a limited number of accredited investors and qualified institutional buyers in reliance upon exemptions from the registration requirements pursuant to Section 4(a)(2) under the Securities Act and Regulation D promulgated under the Securities Act. Individuals who purchased securities as described above represented their intention to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were affixed to the share certificates issued in such transactions.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits.
| Exhibit Number |
Description of Exhibit | |
| 1.1* | Form of Underwriting Agreement | |
| 3.1 | Amended & Restated Certificate of Incorporation of Pasithea Therapeutics Corp. | |
| 3.2 | Bylaws of Pasithea Therapeutics Corp. | |
| 4.1* | Specimen Common Stock Certificate evidencing the shares of Common Stock | |
| 5.1* | Opinion of McDermott Will & Emery LLP | |
| 10.1 | Zen Knightsbridge and Holborn Collaboration Agreement | |
| 10.2 | Zen Baker and Portman Collaboration Agreement | |
| 10.3 | Form of Professional Corporation Agreement | |
| 10.4 | IV Docs Subcontract Agreement | |
| 10.5 | Employment Agreement between Pasithea Therapeutics Corp. and Dr. Tiago Reis Marques | |
| 21.1* | Subsidiaries of the Registrant | |
| 23.1 | Consent of Independent Registered Public Accounting Firm (Marcum LLP) | |
| 23.2* | Consent of McDermott Will & Emery LLP (included in Exhibit 5.1) | |
| 24.1* | Power of Attorney (included on signature page) |
| * | To be filed by amendment. |
(b) Financial Statement Schedules. Schedules not listed above have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
Item 17. Undertakings.
The undersigned registrant hereby undertakes to provide to the underwriter, at the closing specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned hereby undertakes that:
| (1) | For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| (2) | For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-3
SIGNATURES
Pursuant to the requirements of the Securities Act, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Miami, State of Florida, on this 13th day of April, 2021.
| PASITHEA THERAPEUTICS CORP. | ||
| By: | /s/ Dr. Tiago Reis Marques | |
| Dr. Tiago Reis Marques | ||
| Chief Executive Officer and Director | ||
SIGNATURES AND POWER OF ATTORNEY
We, the undersigned officers and directors of Pasithea Therapeutics Corp., hereby severally constitute and appoint Dr. Tiago Reis Marques and Dr. Yassine Bendiabdallah, and each of them singly (with full power to each of them to act alone), our true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution in each of them for him and in his name, place and stead, and in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement (or any other registration statement for the same offering that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act of 1933), and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as full to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or any of them, or their or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities held on the dates indicated.
| Signature | Title | Date | ||
| /s/ Dr. Tiago Reis Marques | Chief Executive Officer and Director | April 13, 2021 | ||
| Dr. Tiago Reis Marques | (principal executive officer) | |||
| /s/ Stanley M. Gloss | Chief Financial Officer | April 13, 2021 | ||
| Stanley M. Gloss | (principal financial and accounting officer) | |||
| /s/ Dr. Yassine Bendiabdallah | Chief Operating Officer and Director | April 13, 2021 | ||
| Dr. Yassine Bendiabdallah | (principal operating officer) | |||
| /s/ Prof. Lawrence Steinman | Director | April 13, 2021 | ||
| Prof. Lawrence Steinman | ||||
| /s/ Simon Dumesnil | Director | April 13, 2021 | ||
| Simon Dumesnil |
II-4